Spontaneous Necker-cube reversals may not be that spontaneous
- PMID: 37323933
- PMCID: PMC10268006
- DOI: 10.3389/fnhum.2023.1179081
Spontaneous Necker-cube reversals may not be that spontaneous
Abstract
Introduction: During observation of the ambiguous Necker cube, our perception suddenly reverses between two about equally possible 3D interpretations. During passive observation, perceptual reversals seem to be sudden and spontaneous. A number of theoretical approaches postulate destabilization of neural representations as a pre-condition for reversals of ambiguous figures. In the current study, we focused on possible Electroencephalogram (EEG) correlates of perceptual destabilization, that may allow prediction of an upcoming perceptual reversal.
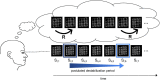
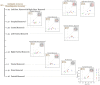
Methods: We presented ambiguous Necker cube stimuli in an onset-paradigm and investigated the neural processes underlying endogenous reversals as compared to perceptual stability across two consecutive stimulus presentations. In a separate experimental condition, disambiguated cube variants were alternated randomly, to exogenously induce perceptual reversals. We compared the EEG immediately before and during endogenous Necker cube reversals with corresponding time windows during exogenously induced perceptual reversals of disambiguated cube variants.
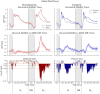
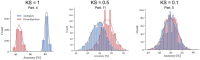
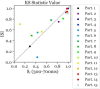
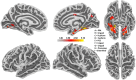
Results: For the ambiguous Necker cube stimuli, we found the earliest differences in the EEG between reversal trials and stability trials already 1 s before a reversal occurred, at bilateral parietal electrodes. The traces remained similar until approximately 1100 ms before a perceived reversal, became maximally different at around 890 ms (p = 7.59 × 10-6, Cohen's d = 1.35) and remained different until shortly before offset of the stimulus preceding the reversal. No such patterns were found in the case of disambiguated cube variants.
Discussion: The identified EEG effects may reflect destabilized states of neural representations, related to destabilized perceptual states preceding a perceptual reversal. They further indicate that spontaneous Necker cube reversals are most probably not as spontaneous as generally thought. Rather, the destabilization may occur over a longer time scale, at least 1 s before a reversal event, despite the reversal event as such being perceived as spontaneous by the viewer.
Keywords: EEG; Necker cube; ambiguous figures; bistable perception; perceptual reversals.
Copyright © 2023 Wilson, Hecker, Joos, Aertsen, Tebartz van Elst and Kornmeier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- American Clinical Neurophysiology Society (2006). Guideline 5: Guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 23 107–110. - PubMed
-
- Atmanspacher H. (1992). Categoreal and acategoreal representation of knowledge. Cogn. Sys. 3 259–288.
-
- Atmanspacher H., Bach M., Filk T., Kornmeier J., Römer H. (2008). Cognitive time scales in a necker-zeno model for bistable perception. Open Cybernet. Syst. J. 2 234–251.
Grant support
LinkOut - more resources
Full Text Sources
