Abstract
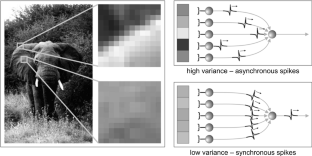
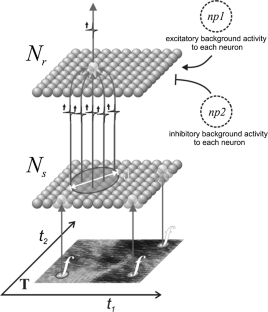
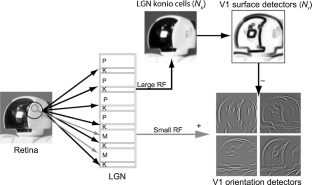
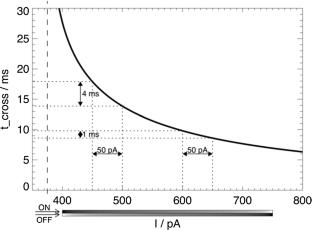
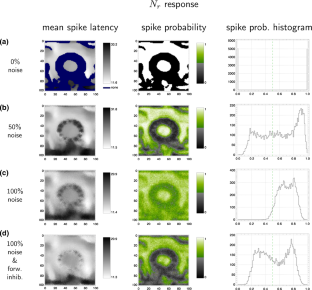
In studies of the visual system as well as in computer vision, the focus is often on contrast edges. However, the primate visual system contains a large number of cells that are insensitive to spatial contrast and, instead, respond to uniform homogeneous illumination of their visual field. The purpose of this information remains unclear. Here, we propose a mechanism that detects feature homogeneity in visual areas, based on latency coding and spike time coincidence, in a purely feed-forward and therefore rapid manner. We demonstrate how homogeneity information can interact with information on contrast edges to potentially support rapid image segmentation. Furthermore, we analyze how neuronal crosstalk (noise) affects the mechanism’s performance. We show that the detrimental effects of crosstalk can be partly mitigated through delayed feed-forward inhibition that shapes bi-phasic post-synaptic events. The delay of the feed-forward inhibition allows effectively controlling the size of the temporal integration window and, thereby, the coincidence threshold. The proposed model is based on single-spike latency codes in a purely feed-forward architecture that supports low-latency processing, making it an attractive scheme of computation in spiking neuronal networks where rapid responses and low spike counts are desired.
This is a preview of subscription content, access via your institution.




References
Arieli A, Sterkin A, Grinvald A, Aertsen A (1996) Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273:1868–1871
Borst JGG, Soria Van Hoeve J (2012) The calyx of held synapse: from model synapse to auditory relay. Annu Rev Physiol 74:199–224. https://doi.org/10.1146/annurev-physiol-020911-153236
Bostner Ž, Knoll G, Lindner B (2020) Information filtering by coincidence detection of synchronous population output: analytical approaches to the coherence function of a two-stage neural system. Biol Cybern 114:403–418. https://doi.org/10.1007/s00422-020-00838-6
Braitenberg V, Schüz A (1991) Anatomy of the cortex: statistics and geometry. Springer, Berlin
Chance FS, Abbott LF, Reyes AD (2002) Gain modulation from background synaptic input. Neuron 35:773–782. https://doi.org/10.1016/S0896-6273(02)00820-6
Christie O, Rego J, Jayasuria S (2020) Analyzing sensor quantization of raw images for visual SLAM. In: Proceedings of ICIP 2020, p 2484
Dayan P, Abbott LF (2001) Theoretical neuroscience: Computational and mathematical modeling of neural systems. MIT Press, Cambridge
Delorme A (2003) Early cortical orientation selectivity: how fast inhibition decodes the order of spike latencies. J Comput Neurosci 15:357–365. https://doi.org/10.1023/A:1027420012134
Denman DJ, Contreras D (2016) On parallel streams through the mouse dorsal lateral geniculate nucleus. Front Neural Circuits. https://doi.org/10.3389/fncir.2016.00020
Ding Y, Casagrande VA (1998) Synaptic and neurochemical characterization of parallel pathways to the cytochrome oxidase blobs of primate visual cortex. J Comp Neurol 391:429–443. https://doi.org/10.1002/(SICI)1096-9861(19980222)391:4%3c429::AID-CNE2%3e3.0.CO;2-2
Gewaltig M-O, Diesmann M (2007) NEST (NEural Simulation Tool). Scholarpedia 2:1430
Gewaltig MO, Körner U, Körner E (2003) A model of surface detection and orientation tuning in primate visual cortex. Neurocomputing 52–54:519–524. https://doi.org/10.1016/S0925-2312(02)00767-1
Gollisch T, Meister M (2008) Rapid neural coding in the retina with relative spike latencies. Science 319:1108–1111. https://doi.org/10.1126/science.1149639
Hendry SHC, Reid RC (2000) The koniocellular pathway in primate vision. Annu Rev Neurosci 23:127–153. https://doi.org/10.1146/annurev.neuro.23.1.127
Hendry SHC, Yoshioka T (1994) A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science 264:575–577. https://doi.org/10.1126/science.8160015
Hernández-González A, Cavada C, Reinoso-Suárez F (1994) The lateral geniculate nucleus projects to the inferior temporal cortex in the macaque monkey. NeuroReport 5:2693–2696. https://doi.org/10.1097/00001756-199412000-00071
Hochstein S, Ahissar M (2002) View from the top: hierarchies and reverse hierarchies in the visual system. Neuron 36:791–804. https://doi.org/10.1016/S0896-6273(02)01091-7
Irvin GE, Norton TT, Sesma MA, Casagrande VA (1986) W-like response properties of intralaminar zone cells in the lateral geniculate nucleus of a primate (Galago crassicaudatus). Brain Res 362:254–270
Ito J, Maldonado P, Singer W, Grün S (2011) Saccade-related modulations of neuronal excitability support synchrony of visually elicited spikes. Cereb Cortex 21:2482–2497. https://doi.org/10.1093/cercor/bhr020
Itti L, Koch C (2000) A saliency-based search mechanism for overt and covert shifts of visual attention. Vis Res 40:1489–1506. https://doi.org/10.1016/S0042-6989(99)00163-7
Jayakumar J, Roy S, Dreher B, Martin PR, Vidyasagar TR (2013) Multiple pathways carry signals from short-wavelength-sensitive (‘blue’) cones to the middle temporal area of the macaque. J Physiol 591:339–352. https://doi.org/10.1113/jphysiol.2012.241117
Johansson RS, Birznieks I (2004) First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci 7:170–177. https://doi.org/10.1038/nn1177
Klein C, Evrard HC, Shapcott KA, Haverkamp S, Logothetis NK, Schmid MC (2016) Cell-targeted optogenetics and electrical microstimulation reveal the primate koniocellular projection to supra-granular visual cortex. Neuron 90:143–151. https://doi.org/10.1016/j.neuron.2016.02.036
Körner E, Gewaltig M-O, Körner U, Richter A, Rodemann T (1999) A model of computation in neocortical architecture. Neural Netw 12:989–1005. https://doi.org/10.1016/S0893-6080(99)00049-0
Kremkow J, Aertsen A, Kumar A (2010a) Gating of signal propagation in spiking neural networks by balanced and correlated excitation and inhibition. J Neurosci 30:15760–15768. https://doi.org/10.1523/JNEUROSCI.3874-10.2010
Kremkow J, Perrinet LU, Masson GS, Aertsen A (2010) Functional consequences of correlated excitatory and inhibitory conductances in cortical networks. J Comput Neurosci 28:579–594. https://doi.org/10.1007/s10827-010-0240-9
Kremkow J, Perrinet LU, Monier C, Alonso J-M, Aertsen A, Frégnac Y et al (2016) Push-pull receptive field organization and synaptic depression: mechanisms for reliably encoding naturalistic stimuli in V1. Front Neural Circuits 10:37. https://doi.org/10.3389/fncir.2016.00037
Kuhn A, Aertsen A, Rotter S (2004) Neuronal integration of synaptic input in the fluctuation-driven regime. J Neurosci 24:2345–2356. https://doi.org/10.1523/JNEUROSCI.3349-03.2004
Kupper R, Gewaltig M-O, Körner U, Körner E (2005) Spike-latency codes and the effect of saccades. Neurocomputing 65–66:189–194. https://doi.org/10.1016/j.neucom.2004.10.006
Lagorce X, Orchard G, Galluppi F, Shi BE, Benosman RB (2017) HOTS: a hierarchy of event-based time-surfaces for pattern recognition. IEEE Trans Pattern Anal Mach Intell 39:1346–1359. https://doi.org/10.1109/TPAMI.2016.2574707
Lapicque L (1907) Recherches quantitatives sur l’excitation électrique des nerfs traitée comme une polarization. J Physiol Pathol générale 9:620–635
Livingstone M, Hubel D (1988) Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240:740–749. https://doi.org/10.1126/science.3283936
Lund JS, Yoshioka T, Levitt JB (1994) Substrates for interlaminar connections in area V1 of Macaque monkey cerebral cortex. In: Peters A, Rockland KS (eds) Cerebral cortex: primary visual cortex in primates. Plenum Press, New York, pp 37–60. doi:https://doi.org/10.1007/978-1-4757-9628-5_2.
Masquelier T, Thorpe SJ (2007) Unsupervised learning of visual features through spike timing dependent plasticity. PLoS Comput Biol 3:e31. https://doi.org/10.1371/journal.pcbi.0030031
Morand S, Thut G, De Peralta RG, Clarke S, Khateb A, Landis T et al (2000) Electrophysiological evidence for fast visual processing through the human koniocellular pathway when stimuli move. Cereb Cortex 10:817–825. https://doi.org/10.1093/cercor/10.8.817
Müller NG, Kleinschmidt A (2004) The attentional ‘spotlight’s’ penumbra: center-surround modulation in striate cortex. NeuroReport 15:977–980. https://doi.org/10.1097/00001756-200404290-00009
Nelken I, Chechik G, Mrsic-Flogel TD, King AJ, Schnupp JWH (2005) Encoding stimulus information by spike numbers and mean response time in primary auditory cortex. J Comput Neurosci 19:199–221. https://doi.org/10.1007/s10827-005-1739-3
Niemeyer JE, Paradiso MA (2018) Saccade-based termination responses in macaque V1 and visual perception. Vis Neurosci 35:E025. https://doi.org/10.1017/S0952523818000032
Reinagel P, Reid RC (2000) Temporal coding of visual information in the Thalamus. J Neurosci 20:5392–5400. https://doi.org/10.1523/JNEUROSCI.20-14-05392.2000
Sclar G, Maunsell JHR, Lennie P (1990) Coding of image contrast in central visual pathways of the macaque monkey. Vis Res 30:1–10. https://doi.org/10.1016/0042-6989(90)90123-3
Sincich LC, Park KF, Wohlgemuth MJ, Horton JC (2004) Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 7:1123–1128. https://doi.org/10.1038/nn1318
Solomon SG, Tailby C, Cheong SK, Camp AJ (2010) Linear and nonlinear contributions to the visual sensitivity of neurons in primate lateral geniculate nucleus. J Neurophysiol. https://doi.org/10.1152/jn.01118.2009
Solomon SG, White AJ, Martin PR (1999) Temporal contrast sensitivity in the lateral geniculate nucleus of a New World monkey, the marmoset Callithrix jacchus. J. Physiol. 517(3):907–917. https://doi.org/10.1111/j.1469-7793.1999.0907s.x
Szmajda BA, Buzás P, FitzGibbon T, Martin PR (2006) Geniculocortical relay of blue-off signals in the primate visual system. Proc Natl Acad Sci USA 103:19512–19517. https://doi.org/10.1073/pnas.0606970103.
Tailby C, Solomon SG, Dhruv NT, et al (2007) A new code for contrast in the primate visual pathway. J Neurosci 27:3904–3909. https://doi.org/10.1523/JNEUROSCI.5343-06.2007
Thomson AM, Lamy C (2007) Functional maps of neocortical local circuitry. Front Neurosci 1:19–42. https://doi.org/10.3389/neuro.01.1.1.002.2007
Thorpe S, Fize D, Marlot C (1996) Speed of processing in the human visual system. Nature 381:520–522. https://doi.org/10.1038/381520a0
Thorpe SJ, Imbert M (1989) Biological constraints on connectionist models. In: Pfeifer R, Fogelman-Soulié F (eds) Connectionism in perspective. Elsevier, Amsterdam, pp 63–92
Tsodyks MV, Markram H (1997) The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci 94:719–723. https://doi.org/10.1073/pnas.94.2.719
Tuckwell HC (1988) Introduction to theoretical neurobiology. Cambridge University Press, Cambridge. doi:https://doi.org/10.1017/cbo9780511623271
Ullman S (2000) High-level vision: object recognition and visual cognition. MIT Press, Cambridge
Van Rullen R, Thorpe SJ (2001) Rate coding versus temporal order coding: what the retinal ganglion cells tell the visual cortex. Neural Comput 13:1255–1283. https://doi.org/10.1162/08997660152002852
VanRullen R, Guyonneau R, Thorpe SJ (2005) Spike times make sense. Trends Neurosci 28:1–4. https://doi.org/10.1016/j.tins.2004.10.010
White AJR, Solomon SG, Martin PR (2001) Spatial properties of koniocellular cells in the lateral geniculate nucleus of the marmoset Callithrix jacchus. J Physiol 533:519–535. https://doi.org/10.1111/j.1469-7793.2001.0519a.x
Xu X, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande VA (2001) A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). J Physiol 531:203–218. https://doi.org/10.1111/j.1469-7793.2001.0203j.x
Acknowledgements
We thank Dr. Ursula Körner for her help and support in compiling the evidence on koniocellular processing strategies across primate species that was instrumental in forming the hypothesis underlying this study. This work was supported by BMBF Grant 01GQ0420 to BCCN Freiburg and EU H2020 Grant 945539 (Human Brain Project SGA3) to the University of Hertfordshire.
Author information
Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by Benjamin Lindner.
Rights and permissions
About this article
Cite this article
Schmuker, M., Kupper, R., Aertsen, A. et al. Feed-forward and noise-tolerant detection of feature homogeneity in spiking networks with a latency code. Biol Cybern 115, 161–176 (2021). https://doi.org/10.1007/s00422-021-00866-w
Received:
Accepted:
Published:
Issue Date:
