Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG
- a Neurobiology and Biophysics, Faculty of Biology, University of Freiburg, Schänzlestrasse 1, 79104 Freiburg, Germany
- b Epilepsy Center, University Medical Center Freiburg, Breisacher Straße 64, 79106 Freiburg, Germany
- c Bernstein Center Freiburg, University of Freiburg, Hansastr. 9A, 79104 Freiburg, Germany
- Accepted 3 May 2013
- Available online 16 May 2013
Highlights
- •
Intracranial heartbeat-evoked potentials (HEPs) exist in primary somatosensory cortex.
- •
Intracranial cardiac field artifacts (CFAs) and pulsatility artifacts are widespread.
- •
Heartbeat-related phase-synchrony may be misleading for connectivity analysis.
- •
Heartbeat-related phase-synchrony can be reduced by suitable correction algorithms.
- •
Intracranial EEG is a promising approach to study heartbeat-related neural processing.
Abstract
The perception of one's own heartbeat is a fundamental interoceptive process that involves cortical and subcortical structures. Yet, the precise spatiotemporal neuronal activity patterns underlying the cortical information processing have remained largely elusive. Although the high temporal and spatial resolution of electrocorticographic (ECoG) recordings is increasingly being exploited in functional neuroimaging, it has not been used to study heart cycle-related effects. Here, we addressed the capacity of ECoG to characterize neuronal signals within the cardiac cycle, as well as to disentangle them from heart cycle-related artifacts. Based on topographical distribution and latency, we identified a biphasic potential within the primary somatosensory cortex, which likely constitutes a heartbeat-evoked potential (HEP) of neuronal origin. We also found two different types of artifacts: i) oscillatory potential changes with a frequency identical to the heart pulse rate, which probably represent pulsatility artifacts and ii) sharp potentials synchronized to the R-peak, corresponding to the onset of ventricular contraction and the cardiac field artifact (CFA) in EEG. Finally, we show that heart cycle-related effects induce pronounced phase-synchrony patterns in the ECoG and that this kind of correlation patterns, which may confound ECoG connectivity studies, can be reduced by a suitable correction algorithm. The present study is, to our knowledge, the first one to show a focally localized cortical HEP that could be clearly and consistently observed over subjects, suggesting a basic role of primary sensory cortex in processing of heart-related sensory inputs. We also conclude that taking into account and reducing heart cycle-related effects may be advantageous for many ECoG studies, and are of crucial importance, particularly for ECoG-based connectivity studies. Thus, in summary, although ECoG poses new challenges, it opens up new possibilities for the investigation of heartbeat-related viscerosensory processing in the human brain.
Abbreviations
- CFA, cardiac field artifact;
- CSF, cerebrospinal fluid;
- ECoG, electrocorticogram;
- EEG, electroencephalogram;
- EMG, electromyogram;
- ESM, electrical stimulation mapping;
- FDR, false discovery rate;
- HEP, heartbeat-evoked potential;
- PSI, phase synchrony index;
- SNR, signal-to-noise-ratio
Keywords
- Heartbeat-evoked potential;
- Somatosensory cortex;
- Electrocorticogram;
- Phase synchrony;
- Connectivity;
- Signal quality
Figures and tables from this article:

Fig. 1. Template correction a) Exemplary oscillatory potentials (median in white and 100 individual trials in color) recorded with strip electrode IHC-4 of P1. b) Corrected heart cycle-related potentials after subtracting the median potential from every single trial. Colored lines: individual trials; white line: median over trials. The oscillatory pattern of the averaged heart cycle-related potentials recorded with strip electrode IHC-4 expectedly disappeared after artifact correction.
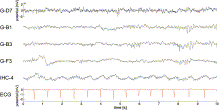
Fig. 2. Ongoing ECoG/ECG recordings (P1); example of ongoing recordings from subdurally-implanted grid electrodes (electrode labels starting with G-) and from one inter-hemispherically-implanted strip electrode (IHC-4) is shown. No clear heart cycle-related potential changes were evident in the ECoG traces of the grid electrodes, but recordings from the strip electrode IHC-4 showed clear persistent oscillatory potential changes with a frequency matching the pulse rate. Amplitude scale of the topmost trace applies to all ECoG traces.
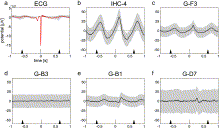
Fig. 3. Trial-averaged potential changes in ECoG/ECG recordings (P1) relative to the ECG R peak. At the bottom of each panel, the median time points of the preceding and subsequent R-peaks are depicted by the tip of the black triangles. The pulse variability (25th to 75th percentile) is indicated by the length of the triangle's base. Red curve (a) and black curves (b–f) represent the average heart cycle-related potential as the median over trials (2270 trials); gray bands extend from the 25th to the 75th percentile of the data; a) ECG potential changes b) potential changes recorded with the inter-hemispherically-implanted strip electrode IHC-4 c–f) potential changes recorded with grid electrodes G-F3, G-B3 and G-B1 located over the left frontal cortex and G-D7 located over the left primary somatosensory cortex of P1 (see fig. 4).

Fig. 4. Heart cycle-related ECoG potential changes: oscillatory potential changes matching the heart pulse rate and late biphasic potentials (P1). a) Location of the electrode-grid of P1 superimposed on a standard brain surface. Circles represent the electrode contacts as determined from a structural MRI scan. Black circles represent electrode contacts that were dropped from the analysis due to poor contact to the cortical surface. b) Averaged potentials for each electrode contact; the scale in the lower left corner of the plot applies to all ECoG traces in the figure. The course of the central sulcus (CS) and the post-central sulcus (PCS) in relation to the electrode positions is depicted by gray lines. High amplitude oscillatory potential changes (examples highlighted by gray rectangles) were widely distributed and had different peak latencies (represented by arrowheads). Biphasic potential changes (highlighted by gray circles) were recorded between CS and PCS (G-C7, G-C8, G-D7 and G-E7) and nearby (G-D8). c) Magnification of the biphasic potential changes in ECoG electrode contact G-D7 (marked with gray asterisk in (b)).
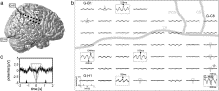
Fig. 5. Heart cycle-related ECoG potential changes: oscillatory potential changes matching the heart pulse rate and sharp peaks (P4). As in P1 (Fig. 4), high amplitude oscillatory potential changes were widely distributed (highlighted by gray rectangles). A few electrodes were contaminated with the CFA, either with a positive peak at time point zero (highlighted by gray triangles) or with a negative peak (highlighted by inverted gray triangles). c) Magnification of the CFA of ECoG electrode contact G-G8 (marked with gray asterisk in (b)); all conventions as in Fig. 4; black circles represent defective electrode contacts.
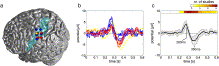
Fig. 6. The delayed biphasic heart cycle-related potential. On the left side (a), electrode contacts that recorded the biphasic potential (represented as colored circles) are shown superimposed on a standard brain surface; blue, red and yellow circles correspond to electrode contacts of P1, P2, and P3, respectively (in P3, the electrode position was mirrored to the left hemisphere for better inter-individual comparison); the transparent blue area indicates the left somatosensory cortex, as determined from the probabilistic maps from the Anatomy Toolbox 1.6 in SPM5. b) Time course of all biphasic potentials at the electrode contacts shown in (a); color coded as in (a); c) Grand average over all selected electrodes; the black curve represents the average potential as the mean over electrodes; the corresponding standard deviation is coded as gray band. The HEP latency of all 12 EEG studies from Table 2 is shown at the top of the graph. The absolute number of studies reporting HEP occurrence at the corresponding point in time is color coded. The latency of the biphasic heart cycle-related potential is in good accordance with the existing literature about HEP latency in EEG recordings.
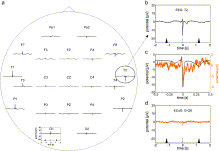
Fig. 7. Heart cycle-related non-invasive EEG potential changes (P4). The averaged potential for each EEG electrode contact is shown in (a); the scale of the electrode O1 in the lower left of the plot applies to all electrode contacts in (a). The timing of the preceding and subsequent R-peaks is illustrated as in Fig. 4; b) magnification of the potential recorded at EEG electrode contact T2; c) black curve represents scale-up of the potential changes recorded with EEG electrode contact T2; orange curve represents the superimposed time course of the simultaneously-recorded ECoG potentials at grid electrode G-G8; d) median potential recorded at ECoG grid electrode contact G-G8; The time course of the potential recorded with grid electrode G-G8 is very similar to the time course of the potential recorded with EEG electrode contact T2 during the QR segment of the cardiac cycle.

Fig. 8. Intra-operatively-acquired photograph. View of the cortical surface after (a) and before electrode placement (b) in P5. In (b), black circles indicate the location of the electrode contacts on the brain surface and relative to the subdural blood vessels. c) Average potential as a median for each electrode contact. The dark red line marks the course of the vena anastomotica inferior (Labbé). Slow oscillatory potential changes were recorded independently of electrode position in relation to the Labbé.

Fig. 9. Spectral composition of an exemplary oscillatory potential matching the heart pulse rate (P4). a) R-peak triggered potential recorded with electrode contact TBA-4 of P4; black curves represent the averaged pulse-related potential as the median over trials; gray bands extend from the 25th to the 75th percentile of the data; b) black curve represents scale-up of the CFA recorded with electrode contact TBA-4; red curve represents the superimposed time course of the simultaneously-recorded ECG channel; c) spectral composition of the signal with relative power, color coded from 0.9 to 1.1; d–f) corresponding frequency-band-power changes for (d) very low (0–12 Hz), (e) high (100–200) and (f) very high (200–400 Hz) frequency bands; colored bands extend from the 25th to the 75th percentile of the data. In summary, the electrode contact TBA-4 recorded pronounced potential changes (oscillatory potential changes matching the pulse rate and a sharp peak at 0 s) as well as pronounced spectral power changes.
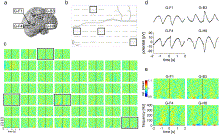
Fig. 10. Topography of heart cycle-related spectral power changes (P4). a) Location of the electrode-grid superimposed on the brain surface of the implanted patient; b) averaged heart cycle-related potentials (same as in Fig. 5). c) Relative spectral power changes for all active grid electrodes in the same time range as in (b) and color coded from 0.9 to 1.1. Black squares indicate the location of electrode contacts that recorded the slow oscillatory potential changes with high peak-to-peak amplitude. Averaged potentials (d) and corresponding relative spectral power changes (e) recorded with the selected grid electrodes G-F1, G-B3, G-F4 and G-H8 are shown in detail. Pronounced spectral power changes even in the high frequency domain accompanied the slow oscillatory potential changes.
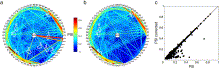
Fig. 11. Heart cycle-related phase synchrony in the very low frequency range (1.5 to 12 Hz) in P1. a) Phase synchrony in the very low frequency range (1.5 to 12 Hz) in a time window of 100 ms to 280 ms after the ECG R-peak between recordings from ECoG electrodes (on the circular line) and an electrode recording the ECG signal (in the center of the circle). Value of phase synchrony between electrode pairs is coded with phase-synchrony-index (PSI)-dependent colors of the connecting lines between the correspondent electrodes. Higher PSIs are also highlighted by the use of thicker lines. White circles 1–3 highlight the highest PSIs of ECoG channels to the ECG channel, while the circles 4–5 bundle the PSIs between these ECoG channels. 6 and 7 illustrate high PSIs between distant ECoG channels that were not phase-locked to the ECG channel. 8 and 9 highlight high PSIs of nearby ECoG channels; b) PSIs between electrode pairs as in a), but after subtracting the average potential (as the median over trials) from every single trial separately for each electrode. Note that the high PSIs between ECoG electrodes and the ECG channel (white circles 1–3 in a), as well as the high PSIs between these ECoG electrodes (white circles 4–5 in a) decreased. High PSIs between ECG-independent-ECoG electrodes (white circles 6–9) were not affected; c) Overview of all PSIs between ECoG channels; every black dot represents one electrode pair, with x-value corresponding to the original PSIs and the y-value corresponding to the PSIs after the ECG correction.
Table 1. Types of heart cycle-related effects in the EEG.
Timing and spatial distribution in the EEG as described in previous studies.
- View Within Article
Table 2. Previous EEG studies on heartbeat-evoked potentials (HEPs).

Studies are sorted according to their publication date. HEP latencies are relative to the ECG R-peak.
- View Within Article
Copyright © 2013 Elsevier Inc. All rights reserved.