Crippling rhythms of the brain
Network model reveals new insights into causes and possible treatments of Parkinson’s disease
Freiburg, 03.11.2011
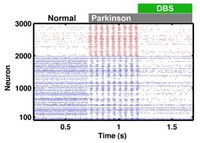
The model explains how the activity of nerve cells in two brain regions (impulses in red and blue) builds up to oscillations in Parkinson’s (grey) due to strong inhibition – and how deep-brain stimulation (DBS, green) can quench it.
Although Parkinson’s disease is one of the most common neurological disorders, its causes at the level of neuronal mechanisms are still poorly understood. Electrical stimulation has proven to alleviate many symptoms, but how exactly it achieves this, remains a mystery. Arvind Kumar and colleagues at the Bernstein Center Freiburg now provide a unified explanation of how Parkinson’s symptoms arise, how deep-brain stimulation (DBS) can counteract them, and how the method can be improved. Their study appears in the current issue of “Frontiers in Systems Neuroscience”.
When James Parkinson described the disease that would later bear his name, he called it “shaking palsy”, pointing at its most obvious symptoms. It would take more than a century to discover that the lack of a messenger molecule in the brain lay at the root of the disease. Yet, this discovery could not explain how the movement disorder actually arises. Meanwhile, scientists found in Parkinson patients that in a specific brain region – the basal ganglia – large groups of nerve cells show a periodic rise and fall in activity. Stimulating the affected areas with electric pulses can quench these oscillations and suppress the disease symptoms. But again, hypotheses how this was achieved were lacking.
Arvind Kumar and colleagues now propose a unified explanation why these oscillations occur and how DBS acts against them. Using a realistic network model, they show that the excessive activity in a certain brain structure – the striatum – forces the basal ganglia into oscillations. In the healthy state, two subregions of the basal ganglia remain in balance through the mutual excitation and inhibition of their activity. However, an increased input from the striatum destroys this balance and the two subregions enter a fatal cycle of rising and falling activity. In other words: Low activity in the striatum quenches the oscillations, high activity unleashes them.
Kumar and colleagues extended their model to understand the mechanism of deep-brain stimulation. Not only does it explain how DBS can return this balance, but also allows investigating improved stimulation patterns. The Freiburg group already proposed a new protocol that uses only half the pulses currently in use. This could double the life span of the DBS implant’s battery, meaning fewer battery replacement surgeries.
Kumar A., Cardanobile S., Rotter S. and Aertsen A. (2011) The role of inhibition in generating and controlling Parkinson’s disease oscillations in the basal ganglia. Front. Syst. Neurosci. 5:86. doi: 10.3389/fnsys.2011.00086
 Drucken
Drucken

