|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
The Journal of Neuroscience, January 28, 2009, 29(4)
This Week in The Journal
This Week in The Journal
Cellular/Molecular
Mutated LRRK2 Activates Cell Death Pathways
Cherry Cheng-Ying Ho, Hardy J. Rideout, Elena Ribe, Carol M. Troy, and William T. Dauer
(see pages 1011–1016)
Among the few familial forms of Parkinson's disease (PD), the most commonly mutated gene is leucine-rich repeat kinase 2 (LRRK2). Based on its subcellular localization, interaction partners, and homologies with other proteins, LRRK2 is thought to play a role in membrane trafficking; but how LRRK2 mutations lead to PD is unknown. Ho et al. hypothesized that LRRK2, like proteins with similar kinase domains, is involved in extrinsically triggered programmed cell death. Activation of this pathway causes the Fas-associated protein with death domain (FADD) to interact with caspase-8, leading ultimately to apoptosis. When transfected into cell lines, mutated LRRK2 interacted with FADD and activated caspase-8. Transfection of a dominant-negative FADD or knockdown of caspase-8 prevented LRRK2-mediated neurodegeneration in mouse cortical cultures. Finally, brains of patients with LRRK2 mutations showed signs of caspase-8 activity, suggesting that this pathway may indeed contribute to the pathogenesis of PD in humans.
Development/Plasticity/Repair
Synaptopodin and GluR1 Expression Are Correlated in Spines
Andreas Vlachos, Eduard Korkotian, Eldi Schonfeld, Ekaterini Copanaki, Thomas Deller, and Menahem Segal
(see pages 1017–1033)
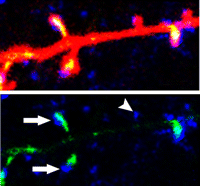
The spine apparatus is an extension of smooth endoplasmic reticulum that passes from the dendritic shaft into large spine heads. The apparatus has been hypothesized to transport proteins to the postsynaptic density and/or to serve as a calcium reservoir. Synaptopodin is an essential component of the spine apparatus. To identify possible roles of synaptopodin, Vlachos et al. expressed GFP-tagged synaptopodin in cultured hippocampal neurons. Approximately 30% of spines—mainly large ones—contained synaptopodin, and these spines had more glutamate receptors and larger glutamate responses than those lacking synaptopodin. Ryanodine receptors, which trigger calcium release from internal stores, were also present predominantly in synaptopodin-expressing spines. Synaptopodin knockdown reduced the percentage of spines that contained ryanodine receptors and blocked LTP-induced increases of glutamate receptors in spines. Because the spine apparatus is absent in mice lacking synaptopodin, whether the observed effects stemmed directly from loss of synaptopodin or resulted from loss of the spine apparatus is not clear.
View larger version (83K):
[in this window]
[in a new window]
More glutamate receptors (blue) are expressed in spines that contain synaptopodin (green, arrows) than in spines that lack synaptopodin (arrowhead). See the article by Vlachos et al. for details.
Behavioral/Systems/Cognitive
Populations of Cortical Neurons Follow High-Frequency Inputs
Clemens Boucsein, Tom Tetzlaff, Ralph Meier, Ad Aertsen, and Björn Naundorf
(see pages 1006–1010)
Cortical neurons receive thousands of excitatory and inhibitory synaptic inputs, which lead to opening and closing of many types of ligand- and voltage-dependent channels distributed throughout the neuronal membrane. Synaptic activity from ascending, descending, and recurrent connections interacts with stochastic channel activity to produce constant fluctuations in membrane potential that resemble random noise when measured in the cell body. How well changes in firing rates can represent a stimulus that arrives amidst this fluctuating noise is a fundamental question in neuroscience. Although theoretical studies suggest neurons can follow extremely high-frequency stimulation, few studies have tested predictions in real neurons, which might filter rapid fluctuations in synaptic signals. Based on recordings from rat cortical neurons, Boucsein et al. report that real neurons represent high-frequency signals remarkably well. The average response of a neuron over many trials (or, by extrapolation, a population of many neurons) could track signals up to many hundreds of cycles per second.
Neurobiology of Disease
Novel AChR in Forebrain Is Highly Sensitive to Amyloid
Qiang Liu, Yao Huang, Fenqin Xue, Alain Simard, Jamie DeChon, Guohui Li, Jianliang Zhang, Linda Lucero, Min Wang, Michael Sierks, Gang Hu, Yongchang Chang, Ronald J. Lukas, and Jie Wu
(see pages 918–929)
Neuronal nicotinic acetylcholine receptors (nAChRs) are pentamers composed of five
subunits or a combination of
and β subunits. The most common nAChR subtypes in the mammalian brain are
4β2 heteropentamers, which regulate dopamine release from striatal neurons, and
7 homopentamers, which regulate glutamate release and have been implicated in learning and memory. Loss of cholinergic neurons in the basal forebrain is an early pathological feature of Alzheimer's disease, and Liu et al. report that cholinergic neurons in rat basal forebrain express β2 and
7 nAChR subunits. Surprisingly, coimmunoprecipitation experiments suggested that these subunits coassemble to form a novel
7β2 nAChR subtype. These novel nAChRs could be distinguished from
7 homopentamers and
4β2 heteropentamers by their unique pharmacological and electrophysiological properties, which were confirmed by heterologous expression in Xenopus oocytes. Most intriguingly,
7β2 nAChRs were highly sensitive to inhibition by amyloid β oligomers, which are linked to Alzheimer's pathology.
Related articles in J. Neurosci.:
- Dynamical Response Properties of Neocortical Neuron Ensembles: Multiplicative versus Additive Noise
- Clemens Boucsein, Tom Tetzlaff, Ralph Meier, Ad Aertsen, and Björn Naundorf
J. Neurosci. 2009 29: 1006-1010.[Abstract] [Full Text] - The Parkinson Disease Protein Leucine-Rich Repeat Kinase 2 Transduces Death Signals via Fas-Associated Protein with Death Domain and Caspase-8 in a Cellular Model of Neurodegeneration
- Cherry Cheng-Ying Ho, Hardy J. Rideout, Elena Ribe, Carol M. Troy, and William T. Dauer
J. Neurosci. 2009 29: 1011-1016.[Abstract] [Full Text] - Synaptopodin Regulates Plasticity of Dendritic Spines in Hippocampal Neurons
- Andreas Vlachos, Eduard Korkotian, Eldi Schonfeld, Ekaterini Copanaki, Thomas Deller, and Menahem Segal
J. Neurosci. 2009 29: 1017-1033.[Abstract] [Full Text] - A Novel Nicotinic Acetylcholine Receptor Subtype in Basal Forebrain Cholinergic Neurons with High Sensitivity to Amyloid Peptides
- Qiang Liu, Yao Huang, Fenqin Xue, Alain Simard, Jamie DeChon, Guohui Li, Jianliang Zhang, Linda Lucero, Min Wang, Michael Sierks, Gang Hu, Yongchang Chang, Ronald J. Lukas, and Jie Wu
J. Neurosci. 2009 29: 918-929.[Abstract] [Full Text]
This Article Full Text (PDF) Submit an eLetter Alert me when this article is cited Alert me when eLetters are posted Alert me if a correction is posted Services Email this article to a friend Related articles in J. Neurosci. Similar articles in this journal Alert me to new issues of the journal Download to citation manager