Copyright © 2008 Elsevier Inc. All rights reserved.
Movement related activity in the high gamma range of the human EEG
Tonio Balla, b, f,  , 1,
, 1,  , Evariste Demandtc, 1, Isabella Mutschlera, d, e, Eva Neitzelb, Carsten Mehringb, c, Klaus Vogtc, Ad Aertsenb, f and Andreas Schulze-Bonhagea, b
, Evariste Demandtc, 1, Isabella Mutschlera, d, e, Eva Neitzelb, Carsten Mehringb, c, Klaus Vogtc, Ad Aertsenb, f and Andreas Schulze-Bonhagea, b
aEpilepsy Center, University Clinics, Albert-Ludwigs-University, Freiburg, Germany
bBernstein Center for Computational Neuroscience, Albert-Ludwigs-University, Freiburg, Germany
cNeurobiology and Animal Physiology, Faculty of Biology I, Albert-Ludwigs-University, Freiburg, Germany
dDepartment of Psychiatry, University of Basel, Basel, Switzerland
eDepartment of Psychology, University of Basel, Basel, Switzerland
fNeurobiology and Biophysics, Faculty of Biology III, Albert-Ludwigs-University, Freiburg, Germany
Received 4 December 2007;
Abstract
Electrocorticographic (ECoG) recordings obtained using intracranially implanted electrodes in epilepsy patients indicate that high gamma band (HGB) activity of sensorimotor cortex is focally increased during voluntary movement. These movement related HGB modulations may play an important role in sensorimotor cortex function. It is however currently not clear to what extent this type of neural activity can be detected using non-invasive electroencephalography (EEG) and how similar HGB responses in healthy human subjects are to those observed in epilepsy patients. Using the same arm reaching task, we have investigated spectral power changes both in intracranial ECoG recordings in epilepsy patients and in non-invasive EEG recordings optimized for detecting HGB activity in healthy subjects. Our results show a common HGB response pattern both in ECoG and EEG recorded above the sensorimotor cortex contralateral to the side of arm movement. In both cases, HGB activity increased around movement onset in the 60–90 Hz range and became most pronounced at reaching movement end. Additionally, we found EEG HGB activity above the frontal midline possibly generated by the anterior supplementary motor area (SMA), a region that was however not covered by the intracranial electrodes used in the present study. In summary, our findings show that HGB activity from human sensorimotor cortex can be non-invasively detected in healthy subjects using EEG, opening a new perspective for investigating the role of high gamma range neuronal activity both in function and dysfunction of the human cortical sensorimotor network.
Article Outline
Neuronal network oscillations may play a basic functional role in voluntary movement ([Salenius and Hari, 2003], [Schnitzler et al., 2006] and [Witham and Baker, 2007]). An important experimental tool for investigating oscillatory brain activity is the electroencephalogram (EEG) recorded from the scalp surface. Movement related changes in the alpha and beta band power of the EEG have been repeatedly described (Neuper and Pfurtscheller, 2001). However, only few cases of movement related modulation of gamma frequency range power in the human EEG have been reported ([Pfurtscheller et al., 1993] and [Pfurtscheller and Neuper, 1992]). In these studies, a narrow frequency band around 40 Hz was investigated, showing enhanced spectral power prior to movement onset and suppression during movement execution.
Movement related oscillations in the 40 Hz range were proposed to be involved in functional interactions between sensorimotor areas during movement preparation (Pfurtscheller et al., 1993), to reflect increased cortical excitability (Aoki et al., 1999), to play a role in focused attention (Bouyer et al., 1987), sensorimotor integration ([Aoki et al., 1999] and [Szurhaj et al., 2005]), or in the neuronal computation of details of movement execution (for a discussion of these hypotheses see Rickert et al., 2005).
Contrasting the ‘40 Hz activity’ described in non-invasively recorded EEG, invasive recordings in epilepsy patients have provided a different picture of movement related gamma oscillations: in particular in the pre- and postcentral gyrus, gamma band responses were found in a highly reproducible way both during externally paced (Crone et al., 1998) and self-paced hand and arm movements ([Ball et al., 2004], [Miller et al., 2007], [Ohara et al., 2000], [Pfurtscheller et al., 2003] and [Szurhaj et al., 2005]). Moreover, all these studies agree that gamma activity was most pronounced during movement execution, instead of during movement preparation. Furthermore, both the low (30–50 Hz) and high (50–150 Hz) gamma bands2 were typically found to show increased spectral power during movement execution.
Recently, there is increasing attention being paid to HGB activity (Crone et al., 2006). Incentives for this new interest were results indicating that HGB is closely correlated to cortical function ([Brovelli et al., 2005] and [Sinai et al., 2005]) and to the blood oxygenation level dependent (BOLD) signal as measured with functional magnetic resonance imaging (fMRI, [Brovelli et al., 2005], [Logothetis et al., 2001], [Mukamel et al., 2005] and [Niessing et al., 2005]). The assumption that HGB activity has functional importance has been further strengthened by evidence from invasive recordings in epilepsy patients, indicating that cross-frequency coupling between theta (4–8 Hz) and HGB activity is involved in the functional coordination of cortical areas in humans (Canolty et al., 2006). The exact role of movement related HGB activity, however, remained elusive. To further investigate this type of brain activity, a non-invasive approach would be highly useful. Thus, the aim of the present study was to re-investigate movement related brain activity in the high gamma band of the EEG of healthy human subjects. To obtain information about the brain regions, time periods, and frequencies where we could expect HGB modulation, we also analyzed electrocorticographic (ECoG) recordings that we obtained in epilepsy patients during the same motor task as used in the EEG experiments.
Methods
EEG experiments
Eight subjects (4 females, 4 males, mean age = 24 years, range = 20–29 years) participated in this study after giving their written informed consent. All participants were without a history of psychiatric or neurological disease, and none had previous experience with a similar experimental setup. Subjects were right-handed according to the Edinburgh handedness questionnaire (Oldfield, 1971): mean = 89%, range = 70–100%.
EEG experiments were carried out in an electrically shielded, dimly lit room. Subjects were seated comfortably in an inclined chair and were instructed to fix their gaze on a fixation point on the wall in front of them (distance approx. 1.5 m). A cape was used to shield the subjects’ arms and shoulders from their field of view to avoid visual guidance and any visual feedback of their arm movements. The cape was peripherally supported assuring that the moving arm of the subjects did not touch the cape. In front of the subjects, five buttons (button diameter: 40 mm) were mounted on a table. The buttons were horizontally arranged in a cross with one central and four peripheral positions. Relative to the central position, the peripheral positions were to the right, left, front, and back. Center-to-center distances between the central and peripheral buttons were 20 cm. A minimal force of 0.06 N had to be applied to push each button.
The recording session was divided in 7 to 11 runs of approx. 10 min duration each. As starting position for each run, the subjects were instructed to rest their hand on the center button and their arm on the supporting table. Due to the low force level required to push the buttons, no active muscle contraction was required to keep the central button depressed, hence, the weight of the hand and arm was sufficient. Subjects were instructed to relax their arm in this position and received feedback from the experimenter if their arm was not properly relaxed (as judged from EMG recordings, see below). Subjects were instructed to perform center-out and center-in arm reaching movements self-paced approximately every 4 to 10 s. Each time when either one of the peripheral targets or the central target was reached after a center-out or a center-in movement, respectively, subjects waited for approximately 4 to 10 s in a relaxed position similar to the starting position described above, before they initiated the next (center-in or center-out) movement. Movement direction of the center-out movements was trial-by-trial self-chosen by the subjects. Triggers generated by releasing and pushing the buttons were used to determine movement onset and movement end, respectively.
Using a 64-channel EEG system (SynAmps, NeuroScan, El Paso, USA), electrical potentials (bandpass filter 0.05–500 Hz) were recorded from 58 standard scalp positions (EASYCAP, Herrsching-Breitbrunn, Germany) equally distributed over both hemispheres. All channels were recorded against a reference electrode at the vertex (CZ). The ground electrode was positioned in the right occipital region. Amplification was 12,500×, sampling frequency was at 2500 Hz. At these settings and given the digital resolution of 16 bit of the amplifier system used, the dynamic range for the scalp EEG channels was 400 µV, i.e. 0.006 µV/bit. Electrode impedances were kept below 5 kΩ. The EMG above the right superficial flexor digitorum longus muscle (pars indicis) and of the right deltoid were recorded in all subjects. The EOG was also recorded to reject trials contaminated with eye movements from further analysis. Amplification of EMG and EOG channels was 2500×. If the dynamic range at this amplification (i.e. 2.2 mV) was too small in respect to EMG amplitude, we used 1000× amplification (dynamic range of 5.5 mV).
ECoG experiments
Two patients (P1: female, aged 55 and P2: male, aged 20) suffering from intractable pharmaco-resistant epilepsy with a focal cortical dysplasia in the left fronto-polar cortex were included in the study after having given their informed consent. The patients were right-handed after a modified Oldfield questionnaire (Oldfield, 1971) and showed no clinical signs of pareses or other movement disorders. The study was approved by the ethics committee of the University Clinics Freiburg. Platinum grid electrodes (4 mm electrode diameter, 112 contacts, 7.1 mm inter-electrode distance) were subdurally implanted above the fronto-parieto-temporal region of the left hemisphere (Fig. 3a). The site of electrode implantation was exclusively based on the requirements of the clinical evaluation. The patients performed a self-paced center-out reaching task with the right arm in four directions (right, left, forward, backward, target distance 20 cm) identical to the task used in the EEG experiments (for minor differences, see Supplementary Methods). Electrocorticograms (ECoG) were recorded using a clinical AC EEG-System (IT-Med, Erlangen, Germany) at 256 Hz (P1) and 512 Hz (P2) sampling rate and 5 s time constant (corresponding to a high-pass filter with 0.032 Hz cutoff frequency) using an intracranial channel as the reference electrode. Onset and end of arm movements were determined based on digital video (25 Hz sampling rate) synchronized to the ECoG. Details on electrical cortical stimulation and on the anatomical assignment of electrode positions are given in the Supplementary Methods.
EEG and ECoG analysis
Both EEG and ECoG data were first re-referenced to a common average reference. EEG trials contaminated with artifacts (excessive EMG, blinks, eye movements, head movements) were discarded from further analysis. From a total number of 820 trials (median across subjects, range: 759–922 trials) a median number of 526 trials per subject (range: 337–591 trials) thus entered the analysis of onset aligned data after artifact rejection. For the analysis of movement end aligned data, a median number of 515.5 trials per subject (range: 313–618 trials) was used. Thus, on average, 36% of the recorded trials were discarded from further analysis.
For the EEG analysis, two sets of data were generated, one using movement onset as triggers and one using movement end, i.e. the time point when the hand reached the target, as triggers. Analysis time windows were from 680 ms before to 560 ms after movement onset and from 560 ms before to 680 ms after movement end. For analysis of the ECoG data, ‘center-out’ and ‘center-in’ trials were also pooled (164 in total in P1, 204 in P2). Analysis time windows were from 1 s before to 3 s after movement onset. The time window for the ECoG analysis was chosen longer than for the EEG, because longer EEG time windows would have resulted in a higher number of EEG trials to be rejected because of contamination by artifacts. In case of the ECoG, there was no significant artifact problem and therefore it was possible to choose a longer time window for analysis without substantially losing data. Time-resolved amplitude spectra of all EEG, ECoG, and EMG channels were calculated for each trial individually by a multi-taper spectral analysis method (Percival and Walden, 2002) using 2 slepian tapers and a time window of 320 ms duration, time step was 40 ms. We computed relative power spectra for each trial in the following way: for each frequency bin, we divided the time-resolved amplitude by the mean baseline amplitude for this frequency. Both for EEG and ECoG, the baseline amplitude was determined as the power in the first analyzed time bin before the arm movement onset. I.e. also the time window around movement end was analyzed relative to the baseline defined before movement onset. Then, the mean across all trials was computed and the Z-scores of the spectral power changes relative to baseline activity were determined for each subject. Spectral changes were considered as significant if the median Z-score across subjects was greater than 3.76, corresponding to a p-value of p < 0.01 that was Bonferroni corrected for the multiple comparisons made for the 59 electrode channels. It is important to note that it was not the aim of this study to test for significant effects for each single bin of the time-frequency plane. Rather, the analysis of the EEG data was guided by the a priori hypotheses derived from the intracranial recordings. In particular, we expected an increase in HGB power in the 60 to 90 Hz range both around movement onset and movement end. On the other hand, evaluation of the whole scalp topography of the EEG was an important aspect of the present study. Therefore we used a correction for multiple comparisons for the 59 electrode channels but not for the individual resolution elements of the whole time-frequency plane. All group averages were based on the median across subjects to prevent group results that could be due to outliers in the individual data.
As an additional analysis, ECoG data was split according to the movement duration (the time from movement onset to movement end) into the half of trials with below median movement duration (“fast” movements) and the half of trials with above median movement duration (“slow” movements). Average spectral power changes were then separately determined for both data sets.
Results
Reaching movement durations showed considerable variability between subjects (median movement duration ranging from 0.43 s to 1.12 s, Table 1). Consequently, transient effects time-locked to movement end might not have been (optimally) detected, if only data aligned to movement onset had been analyzed. Therefore, as described in the Methods section, EEG analyses were carried out both on data aligned to movement onset and to movement end (defined as the point of time when the target button was pressed).
Timing information of arm movement. Median, lower (IQR1), and upper (IQR2) limits of the interquartile range of movement durations for the subjects of the EEG study (S1–S8, sorted according to median movement time)
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |
|---|---|---|---|---|---|---|---|---|
| Median (sec) | 0.43 | 0.44 | 0.47 | 0.65 | 0.66 | 0.75 | 0.76 | 1.12 |
| IQR1 (sec) | 0.39 | 0.35 | 0.38 | 0.55 | 0.59 | 0.65 | 0.68 | 1.01 |
| IQR2 (sec) | 0.48 | 0.65 | 0.58 | 0.81 | 0.77 | 0.89 | 0.84 | 1.24 |
Movement duration was defined as the time interval from releasing the start point until reaching the target.
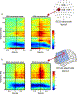
Time-frequency plots of movement related power changes above sensorimotor cortex contralateral to the side of movement are shown for the frequency range from 0.05 to 150 Hz (EEG) and 0.032 to 128 Hz (ECoG) in Fig. 1. Both in the EEG and ECoG, HGB activity increased around movement onset in the 60–90 Hz range and became most pronounced at reaching movement end, extending to frequencies of up to 130 Hz. Furthermore, similar power decreases in the lower frequencies (alpha and beta bands) were evident in both EEG and ECoG results. In both of the patients, we found significantly increased HGB power in motor cortical channels in the 60 to 90 Hz range, both around movement onset and around movement end (p < 0.01). Of the individual EEG subjects, 4 of the 8 subjects showed a significant peak of HGB activity at electrode position C3 at movement onset (i.e. in the time window − 100 ms to 100 ms around movement onset, p < 0.01, corrected) and 6 of the 8 subjects showed a significant peak at C3 at movement end.
 | Full-size image (113K)
High-quality image (1006K) |
Fig. 1. Similarity of movement related EEG and ECoG high gamma band spectral power changes. (a) EEG movement related spectral power changes (median across all subjects). Results are shown for electrode C3 approx. above the primary sensorimotor hand area contralateral to the side of the reaching movements, both for movement onset and end. Overall, HGB increases were most pronounced in the approx. 60 to 85 Hz band as indicated by the black dashed lines. Additionally, power decreases in the lower frequencies (alpha and beta bands) can be seen. A relative power of 1 is identical to no change relative to baseline; values between 0 and 1 correspond to a power decrease and values greater than 1 to a power increase. Scalp positions of the channels C3, C4, CZ, and FZ are marked in the upper inset showing the EEG electrode layout. (b) ECoG movement related spectral power changes in motor cortex channels (mean across 20 channels from 2 patients) with hand or arm motor responses upon electrical cortical stimulation. Positions of motor cortex channels of P1 are marked red in lower inset showing the ECoG electrode layout of patient 1. The same frequency range as in (a) from approx. 60 to 85 Hz is again marked by the black dashed lines. As for the EEG, at movement onset, high gamma band (HGB) power increases predominated in this frequency range. Also as in the EEG, the HGB response at movement end was stronger as compared to movement onset and involved a broader frequency band up to approx. 130 Hz. Additionally, again as in the EEG, power decreases in the lower frequencies (alpha and beta bands) can be seen. Additionally, in the last time bins of the movement end related time window, there is a power rebound in the upper beta range extending to the lower gamma band. Both in the EEG and ECoG plots, the frequency range around 50 Hz is marked by white lines. Little relative power changes were detectable in this range, probably due to line hum. The highest frequency that could be investigated in the ECoG was 128 Hz (due to the sampling frequency of 256 Hz) while EEG results are shown up to 150 Hz.
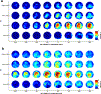
Time-resolved topographies of spectral EEG power changes across the whole gamma band up to 130 Hz are shown in Fig. 2 both for the time window around movement onset (Fig. 2a) and for the time window around movement end (Fig. 2b, median across subjects). In both cases, the most pronounced power increases were within the 60–85 Hz range. Across subjects, significant power increases (p < 0.01, corrected for multiple comparisons, see Methods) first occurred around 240 ms after movement onset. Around movement end, a significant spectral power increase at electrode position C3 (i.e. above the region of left sensorimotor cortex, see Discussion) was present in the frequency range from approx. 30 to 130 Hz. Furthermore, significant spectral power increases both at movement onset and end were also observed above the frontal midline (electrode position FZ).
 | Full-size image (183K)
High-quality image (1368K) |
Fig. 2. Topography of movement related EEG high gamma band activity. (a) Results for the time window around movement onset. Each of the topographic plots (anterior to the top) shows the median scalp distribution of Z-scores of relative power changes, representing a time and frequency bin as indicated on the X- and Y-axes. Electrode positions where the median Z-score corresponded to a p-value < 0.01 (Bonferroni corrected for multiple comparisons for the 59 electrode positions) are marked by white disks. Significant power changes were observed 240 ms after movement onset at electrode position C1, approx. located above the primary sensorimotor arm area, in the frequency bin centered approx. at 72 Hz (59 to 84 Hz) and at electrode position FZ above the frontal midline, presumably recording from the prefrontal cortex or the anterior part of the supplementary motor area (SMA). (b) Results for the time window around movement end. As in Fig. 2a, each of the topographic plots shows the median scalp distribution of Z-scores of relative power changes. Electrode positions with significant modulations (p < 0.01) are marked by white disks. As for movement onset, the most pronounced effects were seen in the 59 to 84 Hz band. In this band, power increases started well before movement end (= the time when the hand reached the target). More broad-band power increases from approx. 30 to 130 Hz were found at electrode position C3 shortly after movement end. Significant effects, particularly in the 59 to 84 Hz band, also extended to regions including the frontal midline.
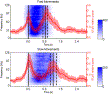
Results of the time-frequency analysis of the ECoG data of P1 together with the functional mapping obtained by direct cortical electrical stimulation are given in Fig. 3. The most pronounced HGB activity was localized at electrode positions recording from the precentral gyrus and showing hand and arm motor responses upon electrical stimulation. Clear modulation of gamma activity reached from approx. 50 Hz up to the highest frequency that could be studied (128 Hz). In several channels, HGB activity showed a biphasic time course such as observed at electrode position E4 in P1 (c.f. Fig. 3a for orientation), located on the precentral gyrus and showing finger movement responses upon electrical stimulation. For this representative ECoG channel, further time-frequency plots are shown in Fig. 4. Data was split according to movement duration in ‘fast movements’ and ‘slow movements’. In both cases, broad-band HGB activity occurred first around movement onset and a second time around movement end, indicating that this biphasic time course was indeed related to movement duration.
 | Full-size image (143K)
High-quality image (1841K) |
Fig. 3. Topography of movement related ECoG high gamma band activity. (a) Position of subdurally implanted electrodes (P1). A square electrode array with 118 contacts (marked blue) was implanted above the left fontal cortex, also covering parts of the anterior parietal and superior temporal lobe. Two additional electrodes stripes were implanted above the anterior temporal lobe and in the fronto-polar region. The electrode positions showing seizure onset, frequent interictal spikes, and rare interictal spikes are color coded. The resection performed subsequently to the invasive diagnostics for treatment of the subject's epilepsy is marked in grey. (b) Time-frequency plots of relative spectral power changes for the part of the ECoG grid covering motor cortex as marked by a red box in (a). Time (x) axis runs from 1 s before to 3 s after movement onset, the frequency (y) axis ranges from 0.032 to 128 Hz; the depicted range of relative power changes is from 0.4 to 3. Plots from primary motor cortex (M1) channels (see Supplementary Methods for the definition of M1 channels used) are marked with black boxes. Letters within the subplots indicate electrical stimulation responses (L — Leg, A — Arm, H — Hand, E — Eye, F — Face; capital letters: motor responses, minor letters: somatosenory responses). Further results from data from the channel marked by an asterix (channel E4) are shown in Fig. 4.
 | Full-size image (51K)
High-quality image (314K) |
Fig. 4. High gamma band ECoG responses and movement duration. Data recorded from channel E4 of P1 located on the precentral gyrus (cf. Fig. 3 for anatomical orientation) were split according to movement duration into fast and slow movements (cf. Methods). In both plots, the first solid black line indicates movement onset and the second solid black line movement end (median across all fast and slow trials, respectively). 25th and 75th percentiles of movement duration are indicated by the dashed lines. Median movement times for the fast and slow trials were approximately 0.8 s and 1.2 s, respectively. The mean time course of HGB spectral power changes (averaged from 50 to 128 Hz) and its standard deviation is shown in red. Additionally, average relative spectral EMG activity for fast and slow movements is shown (white-to-blue colorscale). For both fast and slow movements, a first HGB peak can be seen at movement onset and a distinct, second peak at movement end.
Discussion
Previous electrophysiological studies have addressed the relation of oscillatory brain activity and voluntary movement for the lower frequency components of human electro-encephalographic (EEG) recordings up to the low gamma range around 40 Hz ([Omlor et al., 2007], [Pfurtscheller et al., 1993] and [Pfurtscheller and Neuper, 1992]). High gamma band (HGB) power increases related to self-paced movements in experimental paradigms without any visual cues have been previously demonstrated in invasive recordings in epilepsy patients with subdural recordings ([Ball et al., 2004], [Miller et al., 2007], [Ohara et al., 2000] and [Pfurtscheller et al., 2003]), intracortical recordings (Szurhaj et al., 2005), and using MEG (Dalal et al., 2007). The major results of the present study are that movement related high gamma band activity of up to 130 Hz can be non-invasively detected using scalp recorded EEG in healthy human subjects and that the EEG HGB shows similar response characteristics as HGB activity in intracranial data from epilepsy patients obtained during the same motor task. Furthermore, our results suggest that a major site of origin of the HGB activity seen in EEG is most probably localized to the precentral motor cortex contralateral to the side of movement.
The power density of EEG and local field potential (LFPs) in the mammalian cortex are, overall, inversely proportional to frequency (Freeman et al., 2000). Therefore, gamma band oscillations in general, and the HGB in particular, are of low amplitude compared to alpha and beta band oscillations. Furthermore, the HGB in EEG recordings are prone to artifact contamination, especially caused by muscle activity that can be relatively large in amplitude ([Goncharova et al., 2003] and [Whitham et al., 2007]). Subtle gamma modulations may, therefore, be missed due to their typically low signal-to-noise ratio. In the present study, we optimized our approach for detecting low amplitude HGB activity in several respects. First, we chose a motor task that evokes pronounced HGB enhancement in the human motor cortex (Ball et al., 2008, submitted). Second, because of the probable low amplitude of HGB activity, we recorded EEG at high amplification (× 12,500), similar to a study that was previously successful in detecting gamma bursts up to 150 Hz in a visuo-motor task (Krieger and Dillbeck, 1987). Third, for spectral analysis, we investigated the entire gamma range up to 128 Hz using a multi-tapering method suitable for signal-to-noise improvement (Percival and Walden, 2002). Using this approach we indeed found clear HGB movement related EEG activity both in the group results (median across subjects, Fig. 1 and Fig. 2) and also in the majority of the individual subjects (see also Supplementary Fig. 1).
The 60–85 Hz range where we found movement related HGB EEG modulation lies above the range investigated in previous EEG studies using ‘pure’ motor tasks, but well within the range where invasive studies previously found movement related activity, both in human and monkey motor cortex. However, although investigated, no power changes in the 70–90 Hz range were found in several studies using visuo-motor tasks ([Conway et al., 1995] and [Schoffelen et al., 2005]), presumably reflecting task differences and/or differences in the recording/analysis methods used. On the other side, also using visuo-motor and auditory reaction time tasks, EEG spectral power changes and oscillatory burst activity were previously described up to 150 Hz (e.g. [Gonzalez et al., 2006], [Krieger and Dillbeck, 1987] and [Shibata et al., 1999]). To what extent these results were caused by the visual cues used in these studies, however, is difficult to judge, particularly because HGB activity in the EEG can also be evoked by passive visual stimulation ([Cobb and Dawson, 1960] and [Heinrich and Bach, 2004]) and presumably also during auditory click stimulation (Scheller et al., 2005). Somatotopic patterns of HGB responses during visuo-motor tasks unlikely to result from the visual cues were delineated using ECoG by Crone et al. (1998) and later also by Leuthardt et al. (2007).
Based on the results of our intracranial data obtained using the same movement task as used in the EEG experiment we expected increased HGB power in EEG electrodes recording from the hand and arm representations of the left pre- and postcentral gyrus. In the international 10–10 electrode system, the two electrode positions that are most likely positioned above this area are electrodes C1 and C3 (Okamoto et al., 2004). Indeed, we found a transient, focal enhancement of HGB activity in electrodes C1 and C3 around movement onset and at movement end (Fig. 1 and Fig. 2). An important question is whether these results could possibly be due to EMG contamination. Across subjects, EMG artifacts typically are strongest at the outer electrode positions near face, temporalis, and neck muscles (Goncharova et al., 2003). In contrast, the HGB activity that we have observed was maximal at electrode positions other that these outer electrode positions, lending little support for an EMG origin. Only around movement end, gamma band increases were also observed at peripheral (occipital) electrodes (Fig. 2) indicating a transient EMG contribution in this region, most likely from contraction of neck muscles. Channels with clear HGB increases, including channel C3 as shown in Fig. 1, typically showed a clear decrease in the lower frequencies, i.e. the well known so-called movement related to ‘desynchronization’ (Pfurtscheller et al., [Pfurtscheller and Neuper, 1992] and [Pfurtscheller et al., 2003]). In contrast, EMG is characterized by increased spectral power also in these lower frequencies (Goncharova et al., 2003) and also extends well above 130 Hz, again supporting a neural rather than an EMG origin of the gamma band EEG modulations above sensorimotor cortex described in the present study. Furthermore, a neuronal origin is also strongly indicated by the high similarity of the EEG spectra to those of intracranial data recorded from the same brain region (Fig. 1).
Besides the left central region, HGB enhancement was also observed above the frontal midline including electrode position FZ (Fig. 1 and Fig. 2). The average cortical projection point of standard electrode position FZ (Okamoto et al., 2004) is above the medial prefrontal cortex, approximately 1–2 cm anterior to the probabilistically defined anterior border of area 6 (Eickhoff et al., 2005), indicating that the signals at FZ originated most probably in medial prefrontal cortex or maybe in the anterior part of the supplementary motor area (SMA). Intracranial recordings from these areas during the same movement task would be an important step to further establish and delineate the cortical origin of the HGB effects that we found above the frontal midline.
We analyzed direct cortical (ECoG) recordings from two patients with subdurally implanted electrodes performing the same motor task as used in the EEG experiments. An advantage of ECoG as compared to scalp EEG is that through the close contact to the cortical surface and high spatial resolution of the implanted electrode grid, activity can be mapped with higher reliability and spatial accuracy (Niedermeyer and Lopes da Silva, 2004). Time-frequency analysis of the ECoG data revealed broad-band gamma responses both at movement onset and end (Fig. 1, Fig. 3 and Fig. 4) that included both the low and high bands of the gamma spectrum. Gamma responses were most pronounced at electrode locations on the precentral gyrus that showed hand and arm motor responses upon electrical stimulation. These results suggest that the precentral motor cortex and not the postcentral somatosensory cortex may be the predominant source of the focal gamma band activity observed in the EEG at C3 and C1 electrode positions. To further delineate the location of the EEG sources of movement related HGB activity, however, electrical source analysis techniques ([Ball et al., 1999], [Fuchs et al., 1999] and [Ilmoniemi, 1991]) might be useful.
In respect to their time course, the HGB activity we found both in EEG and ECoG fit well to the previous ECoG observations during visually cued hand movements (Zygierewicz et al., 2005) and intracortical recordings during self-paced finger movements (Szurhaj et al., 2005). In the latter study, increased HGB activity of a single recording channel was either found in the time window around EMG onset or EMG end, but never in both (Szurhaj et al., 2005). This difference to our data and also to the results of Zygierewicz et al. (2005) may be related to the recording techniques (intracortical vs. epicortical), to the movement paradigm (simple finger extension vs. natural, goal-directed arm movement), or to the exact frequency band investigated (40–60 Hz in the work of Szurhaj et al. 2005). More research will be necessary to clarify this issue and to establish, more generally, the relation between motor cortical HGB, muscle activity, and different movement and task parameters.
In respect to their frequency range, the HGB activity we found in the ECoG recordings extended up to the highest frequency that we have investigated, i.e. up to 128 Hz. Pervious intracranial data indicates that movement related HGB modulation in humans extends even further, up to frequencies of 150 to 200 Hz ([Brovelli et al., 2005], [Crone et al., 2006], [Leuthardt et al., 2004] and [Miller et al., 2007]). Movement related HGB responses in the EEG were most pronounced in the range of 60–85 Hz, but our results suggest that they might also extend to even higher frequencies (Fig. 1, Suppl. Fig. 1). Our study was however not designed to statistically evaluate the whole time-frequency plane in detail, but instead we concentrated on an a priori frequency band of interest (60–85 Hz) based on the responses found in the ECoG. Otherwise, the multi comparison problem due to the large number of independent time-frequency resolution elements might have reduced the chance for detecting HGB responses at all. Our results indicate that future studies of movement related EEG changes should not restrict themselves to the range of 60–85 Hz, but might also consider even higher frequencies using statistical methods for assessing changes in the whole time-frequency plane (Zygierewicz et al., 2005).
Further investigations using similar methods as in our study may also probe the role of HGB EEG activity in different sensory systems and in respect to cognitive function ([Crone et al., 2001], [Kaiser et al., 2008], [Lutzenberger et al., 2002] and [Ray et al., 2008]). A further future issue for the exploration of the HGB in EEG arises due to the fact that for a large part we relied on group average results. Some of the individual subjects of the present study did not show significant gamma effects. Either these subjects were really lacking a motor cortical HGB increase or it just remained undetected by the present EEG methods. An argument for the later explanation is the fact that the available intracranial data shows movement related high gamma increases with high inter-individual reproducibility, such as that in all 22 subjects of a recent study (Miller et al., 2007). Further increasing the sensitivity of EEG to gamma band modulation would therefore be a desirable goal. It is currently not clear, to what extent the choice of experimental paradigm, of recoding parameters such as amplification and sampling rate, and of the analysis techniques each contribute to successful detection of HGB activity in the EEG. The importance of each of these factors should be further investigated and might also be further optimized. More reliable within-subject detection of HGB changes would also be necessary for potential diagnostic application of HGB measurement using scalp EEG.
Acknowledgment
The research was supported by the WIN-Kolleg of the Heidelberg Academy of Sciences and Humanities and the German Federal Ministry of Education and Research (BMBF-DIP METACOMP and BMBF grant 01GQ0420 to the BCCN Freiburg).
References
 Corresponding author. Epilepsy Center, University Clinics Freiburg, Hugstetter Str. 49, 79095 Freiburg, Germany.
Corresponding author. Epilepsy Center, University Clinics Freiburg, Hugstetter Str. 49, 79095 Freiburg, Germany.1 Equally contributing authors.
2 Several different definitions of frequency bands above the classical gamma range have recently been proposed: ““high-frequency oscillations”” from 80 to 500 Hz (Staba et al., 2002), ““high gamma frequency”” from 60-200 Hz (Brovelli et al., 2005), ““very high frequency oscillations”” from 80 to 200 HZ (Gonzalez et al., 2006). In the present study we refer to the range from 50 to 150 Hz as ‘‘high gamma band’’.







 E-mail Article
E-mail Article Cited By
Cited By Citation Feed
Citation Feed Export Citation
Export Citation Add to my Quick Links
Add to my Quick Links