
Science,
Vol 273,
Issue 5283,
1868-1871
, 27 September 1996
Dynamics of Ongoing Activity: Explanation of the Large
Variability in Evoked Cortical Responses
Amos Arieli,
Alexander Sterkin,
Amiram Grinvald,
Ad Aertsen
*
Evoked activity in the mammalian cortex and the resulting
behavioral responses exhibit a large variability to repeated
presentations of the same stimulus. This study examined whether the
variability can be attributed to ongoing activity. Ongoing and evoked
spatiotemporal activity patterns in the cat visual cortex were measured
with real-time optical imaging; local field potentials and discharges
of single neurons were recorded simultaneously, by electrophysiological
techniques. The evoked activity appeared deterministic, and the
variability resulted from the dynamics of ongoing activity, presumably
reflecting the instantaneous state of cortical networks. In spite of
the large variability, evoked responses in single trials could be
predicted by linear summation of the deterministic response and the
preceding ongoing activity. Ongoing activity must play an important
role in cortical function and cannot be ignored in exploration of
cognitive processes.
Department of Neurobiology, Weizmann Institute of Science, Post
Office Box 26, Rehovot 76100, Israel.
*
Present address: Department of Neurobiology and Biophysics,
Institute of Biology III, University of Freiburg, Freiburg, Germany.
When a stimulus is presented repeatedly,
the variability of the evoked cortical responses is often as large as
the response itself, both in anesthetized (1) and in awake,
behaving animals (2). The standard approach has been to
adopt a "signal-plus-noise" model, assuming that an individual
evoked response is composed of a reproducible signal added to
uncorrelated noise. The signal is then recovered experimentally from
the noise by averaging over repeated trials (3). This
approach tacitly assumes that variability reflects "noise," which
is a nuisance for cortical processing and could be overcome by the
brain by appropriate averaging over populations of neurons
(4). Numerous articles deal with the question of what the
source of variability in the brain is (5, 6). This issue of
the reliability of cortical responses must be resolved in order to
determine whether the neural code for information transfer in the brain
requires the averaged activity of many neurons (7).
Ongoing cortical activity is far from being just noise (8).
In fact, the spontaneous activity of a single neuron is not an
independent process but is time-locked to the firing or to the synaptic
inputs from numerous other neurons, all activated in a coherent
fashion, even without sensory input. Often the coherent ongoing
activity is as large as evoked activity. Therefore, ongoing activity
must have a major influence on sensory processing. We present evidence
for the hypothesis that cortical evoked activity comprises a
reproducible stimulus response and a dynamically changing ongoing
activity, presumably reflecting varying brain states (9).
We tested the above hypothesis by analyzing the spatiotemporal dynamics
in single-trial responses to visual stimulation (moving gratings).
Experiments were carried out on six anesthetized, muscle-relaxed adult
cats as described elsewhere (8, 10). Activity was measured
in the visual cortex (areas 17 and 18), combining real-time optical
imaging and electrophysiological recordings. A 2-mm-square area of
primary visual cortex, stained with the voltage-sensitive dye RH795,
was imaged onto a 12 × 12 array of photodiodes. Simultaneously,
spike discharges of two isolated neurons and the local field potential
(LFP) were recorded from a microelectrode inserted into the exposed
area. Optical and electrical signals were continuously sampled every
3.5 ms for periods of 70 s.
Real-time optical imaging with the use of voltage-sensitive dyes
measures, at millisecond time resolution, the membrane potential
changes of populations of neuron processes (11). It
emphasizes synaptic input, and hence, the signal is similar to the LFP
(8, 12). We analyzed the dynamics of the nonaveraged
activities in single trials and their organization in space and time
(13, 14). This analysis enabled us to assess the extent to
which individual cortical response patterns are influenced by the
instantaneous network state. Optically recorded images together with
traces of the simultaneously recorded LFP and spike trains are shown in
Fig. 1A for two responses to a repeated visual stimulus. The large
variability revealed in the optically imaged responses
resembles the well-known variability in the LFP and
single-neuron recordings. The fact that the response variability of
synaptic population activity, measured optically and in LFP, is at
least as large as the response itself argues against the assumption
that averaging over local neuron populations would eliminate response
variability (4). Averaging over trials (Fig.
1B) does remove this variability and extracts the
reproducible response. We define time 0 as the moment just before the
onset of the average response. The optically measured activity pattern
at time 0 in an individual trial is here referred to as the initial
state of that trial.
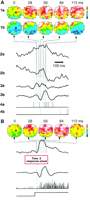
Fig. 1.
Evoked activity in response to repetitive
stimulation exhibits large variability. (A) Two individual
responses (a and b) to a repeated visual stimulus [bottom trace in
(B)]: The images (1a,b) show the activity in a 2 mm by 2 mm area of
cortex, taken at different times from response onset. Activation above
the mean level is coded in red, suppression in blue, as indicated by
the color scale (right); full scale corresponds to a fractional change
of  5 × 10 5 × 10 5). The small square in the first image
marks the site, above the microelectrode, from which the optical traces
(2a,b) were taken. Note the large variability in the evoked response,
also reflected in the LFP (3a,b) and single-neuron spike trains (4a,b),
both recorded simultaneously with the optical signals. The absence of
slow components in the LFP is due to high-pass filtering above 3 Hz.
(B) Average evoked response: The optical images and
signals, LFP, and single-unit activity were averaged, triggered on the
onset of 34 visual stimuli (drifting full-field grating) in the
preferred orientation of the recorded unit.
[View Larger Version of this Image (27K GIF file)] 5). The small square in the first image
marks the site, above the microelectrode, from which the optical traces
(2a,b) were taken. Note the large variability in the evoked response,
also reflected in the LFP (3a,b) and single-neuron spike trains (4a,b),
both recorded simultaneously with the optical signals. The absence of
slow components in the LFP is due to high-pass filtering above 3 Hz.
(B) Average evoked response: The optical images and
signals, LFP, and single-unit activity were averaged, triggered on the
onset of 34 visual stimuli (drifting full-field grating) in the
preferred orientation of the recorded unit.
[View Larger Version of this Image (27K GIF file)]
Searching for systematic rules underlying the response
variability, we found that the evoked activity is highly correlated to
the initial state: The evoked activity is low when the initial state
was low, whereas it is high when the initial state was high. The
relation between the two is approximately linear (Fig.
2A), as expressed by the high correlation coefficient
(R = 0.9, P < 10 12,
n = 34 trials). Such high correlation was found for
most of the recorded area (Fig. 2B) (P < 0.001 in all 35 recording sessions from six cats, each session
containing 34 trials). The correlation was not restricted to the
optical recordings, but held for the electrophysiological recordings as
well. Indeed, the initial state was significantly correlated over a
large area with the evoked LFP (Fig. 2C)
[P < 0.01 in 89% (31/35) of the sessions] and,
albeit to a lower extent, with the single-neuron spike rate (Fig.
2D) [P < 0.01 in 69% (24/35) of the
sessions]. The correlation across the different types of
electrophysiological recordings is expected to be considerably smaller
because they reflect different aspects of cortical activity and
different resolutions in space and time. The optical signal reflects
localized changes in membrane potential, emphasizing synaptic input
restricted to the upper cortical layers. On the other hand, the LFP
reflects the extracellular currents near the electrode tip, with an
ambiguous relation between the amplitude and polarity of the LFP waves
and the brain cell activity in the vicinity of the microelectrode
(15). In the simplest approximation, the LFP is the
derivative of the optical signal. However, both signals are continuous
waves that reflect the activity of thousands of neurons and are
correlated to the state of the animal (16). The action
potentials (spikes), with a time resolution of milliseconds, reflect
the output of single neurons rather than of a population. In view of
these considerations, our findings exhibit a remarkable consistency
across cortical activities at greatly different spatial resolutions,
measured by very different recording techniques. 12,
n = 34 trials). Such high correlation was found for
most of the recorded area (Fig. 2B) (P < 0.001 in all 35 recording sessions from six cats, each session
containing 34 trials). The correlation was not restricted to the
optical recordings, but held for the electrophysiological recordings as
well. Indeed, the initial state was significantly correlated over a
large area with the evoked LFP (Fig. 2C)
[P < 0.01 in 89% (31/35) of the sessions] and,
albeit to a lower extent, with the single-neuron spike rate (Fig.
2D) [P < 0.01 in 69% (24/35) of the
sessions]. The correlation across the different types of
electrophysiological recordings is expected to be considerably smaller
because they reflect different aspects of cortical activity and
different resolutions in space and time. The optical signal reflects
localized changes in membrane potential, emphasizing synaptic input
restricted to the upper cortical layers. On the other hand, the LFP
reflects the extracellular currents near the electrode tip, with an
ambiguous relation between the amplitude and polarity of the LFP waves
and the brain cell activity in the vicinity of the microelectrode
(15). In the simplest approximation, the LFP is the
derivative of the optical signal. However, both signals are continuous
waves that reflect the activity of thousands of neurons and are
correlated to the state of the animal (16). The action
potentials (spikes), with a time resolution of milliseconds, reflect
the output of single neurons rather than of a population. In view of
these considerations, our findings exhibit a remarkable consistency
across cortical activities at greatly different spatial resolutions,
measured by very different recording techniques.
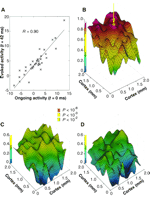
Fig. 2.
Cortical evoked activity is related
to the initial state. (A) Scatter plot of optically
measured evoked activity at a single cortical site 42 ms after response
onset in 34 successive single trials versus the initial state at that
site. Both axes have the same arbitrary units. The straight line
depicts the result of linear regression (correlation coefficient
R = 0.9). (B) Correlation coefficients [as in
(A)] for all sites in the imaged cortical area. The arrow marks the
site, selected in (A). The statistical significance of correlation is
indicated by color. (C) Correlation between the evoked LFP
28 ms after response onset and the initial state. (D)
Correlation between the evoked spike rate, measured over an interval of
35 ms centered around 28 ms after response onset, and the initial
state. The correlations in (C) and (D) are between a single site
(microelectrode recording) and all optically measured
sites.
[View Larger Version of this Image (50K GIF file)]
The high correlations observed in single trials are consistent
with the assumption that the stimulus-evoked activity contains a
reproducible response component and that the changes in the patterns of
evoked activity from trial to trial are caused by the fluctuating
ongoing activity. This view is expressed in a simplified model (Fig.
3A) in which an individual response is the sum of two
components: the reproducible response and the ongoing activity. Thus,
the effect of a stimulus might be likened to the additional ripples
caused by tossing a stone into a wavy sea.

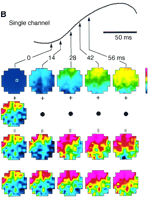
Fig. 3.
Predicting the cortical evoked
response. (A) A single-trial response to a stimulus was
predicted by summing the reproducible response and the ongoing
activity, approximated by the initial state. (B) Comparison
of the predicted and measured responses. (Top trace) Averaged
evoked response (34 trials), measured from a single optical channel
above the microelectrode site (small square in top-left frame). (First
row) Averaged evoked activity pattern (after subtraction of frame 0),
shown at five different times after response onset, indicated by the
arrows. All other rows show single-trial responses. (Second row)
Initial state, approximating ongoing activity during the response.
(Third row) Predicted response, obtained by adding the frames
in the first and second rows. (Fourth row) Measured response.
[View Larger Versions of these Images (68K GIF file)]
A consequence of this simplified model is that we should be able to
predict the response pattern in a single trial by taking into account
the initial state of that trial. This prediction should hold for as
long as the ongoing activity pattern (which presumably continues to
change during the evoked response) is still similar to the initial
state. Given that most of the energy in the LFP is restricted to
frequencies below about 20 Hz, we expect our prediction to perform well
for up to 50 ms after response onset. We calculated the predicted
response by adding the initial state, a single frame (Fig. 3B, second
row), to the averaged response, a series of frames (Fig. 3B, first
row). The result of such prediction (Fig. 3B, third row) corresponds
well to what we actually measured (Fig. 3B, fourth row). We applied
this procedure to all of the data (1190 trials from six cats) and
compared the predicted responses, trial by trial, with the measured
responses.
Particularly good examples of the prediction are shown in Fig.
4A for three consecutive trials in a recording session,
examining the images obtained 28 ms after response onset. Note that the
predictions for different trials vary only in their initial states. The
variability among these initial states (first column) is so large and
the patterns are so heterogeneous that the evoked activity in single
trials (second column) looks very different each time. Yet, in all of
these cases we obtained excellent predictions of the evoked
activity pattern (third column), in spite of the large variability.
Such good predictions were obtained for many of our trials, for periods
of tens of milliseconds after response onset. Subtracting the initial
state (first column) from the measured response (second column) leaves
a net pattern ([M  I], last column): a single-trial estimate
of the reproducible response to this particular stimulus. These net
patterns are very similar, whereas the measured patterns (second
column) are variable, suggesting that "removal" of the ongoing
activity from the measured response does markedly reduce the response
variability. We do not know if the lack of a perfect match among the
net patterns should be attributed solely to the change of ongoing
activity from the initial state or whether, in addition, it reflects
deviations from the simplified, linear model. I], last column): a single-trial estimate
of the reproducible response to this particular stimulus. These net
patterns are very similar, whereas the measured patterns (second
column) are variable, suggesting that "removal" of the ongoing
activity from the measured response does markedly reduce the response
variability. We do not know if the lack of a perfect match among the
net patterns should be attributed solely to the change of ongoing
activity from the initial state or whether, in addition, it reflects
deviations from the simplified, linear model.

Fig. 4.
Quality of prediction of the
response. (A) Three consecutive single-trial responses (1 through 3) to the same visual stimulus, showing the initial state, the
measured response 28 ms later, and the predicted response at that time.
Subtracting the initial state from the measured response yielded the
net pattern [M  I]. (B) Quality of prediction, assessed
by the correlation coefficient between predicted and optically measured
activity patterns as a function of time from response onset. The curve
shows the mean correlation; the error bars denote the standard error of
the mean (n = 35 recording sessions). (C)
Autocorrelation of optically measured activity patterns,
triggered on the response onset (time 0). The
right-hand curve shows the correlation coefficient between the ongoing
activity at time 0 (just before response onset) and the evoked
activity. The left-hand curve shows the correlation coefficient between
the same ongoing activity at time 0 and the ongoing activity before
stimulus onset. After calculating the correlation coefficient for each
pixel in the matrix at a certain delay, we simply summed all the pixels
(because we did not see any consistent temporal differences between the
different pixels). The insets in (B) and (C) show the correlations over
prolonged time.
[View Larger Version of this Image (21K GIF file)] I]. (B) Quality of prediction, assessed
by the correlation coefficient between predicted and optically measured
activity patterns as a function of time from response onset. The curve
shows the mean correlation; the error bars denote the standard error of
the mean (n = 35 recording sessions). (C)
Autocorrelation of optically measured activity patterns,
triggered on the response onset (time 0). The
right-hand curve shows the correlation coefficient between the ongoing
activity at time 0 (just before response onset) and the evoked
activity. The left-hand curve shows the correlation coefficient between
the same ongoing activity at time 0 and the ongoing activity before
stimulus onset. After calculating the correlation coefficient for each
pixel in the matrix at a certain delay, we simply summed all the pixels
(because we did not see any consistent temporal differences between the
different pixels). The insets in (B) and (C) show the correlations over
prolonged time.
[View Larger Version of this Image (21K GIF file)]
To quantify the performance of the prediction, we measured the
correlation coefficient between predicted and measured response
patterns as a function of time from response onset for all the data
(Fig. 4B). The long-lasting high correlation shows that
a deterministic response added to a varying initial state does indeed
approximate the varying individual response. Not surprisingly, the
quality of the prediction declines with time from response onset. This
decline occurs because the prediction procedure (Fig.
3B) reduces the ongoing activity dynamics to a single
snapshot (the initial state). Specifically, it does not take into
account that the ongoing activity continues to change while the evoked
response unfolds. Evidently, we cannot directly measure the ongoing
pattern during that time. We could estimate the expected time course of
this change, however, by determining the autocorrelation of the
optically measured activity patterns, triggered on the response onset
(Fig. 4C). The left-hand part of the graph describes
the statistical behavior of the ongoing activity up to the moment of
response onset, and the right-hand part shows the statistical behavior
of the activity after the initiation of the response. Clearly, the
background ongoing activity has a very similar time course to the
evoked activity (the evoked activity lasted for 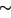 100 ms). In fact,
the remarkable similarity between the two halves of the graph indicates
that, on average, the ongoing dynamics are not affected by the
response. The excellent resemblance between the curve in Fig. 4B and
the left-hand part of Fig. 4C shows that the gradual decline in the
quality of prediction can indeed be attributed to the progressing
deviation of the ongoing activity from the initial state (the curve in
Fig. 4B and the right-hand part of Fig. 4C are identical
mathematically). 100 ms). In fact,
the remarkable similarity between the two halves of the graph indicates
that, on average, the ongoing dynamics are not affected by the
response. The excellent resemblance between the curve in Fig. 4B and
the left-hand part of Fig. 4C shows that the gradual decline in the
quality of prediction can indeed be attributed to the progressing
deviation of the ongoing activity from the initial state (the curve in
Fig. 4B and the right-hand part of Fig. 4C are identical
mathematically).
The brain often does not respond in the same way to a repeated
stimulus, even though cortical neurons are able to respond with
remarkable temporal accuracy (5, 17). Because of this
variability, found also in awake, behaving monkeys
(2), it has been assumed that the signal is
contaminated by the brain's "noise." Our findings provide
experimental evidence to support the hypothesis that the processing of
sensory input in the visual cortex involves the combination of a
deterministic response and ongoing network dynamics. The relation
between ongoing activity and evoked response in first approximation is
linear (18). The combination of these components accounts
for the large response variability in individual trials. It is well
established that the ongoing activity measured by the
electroencephalogram (EEG) is correlated to behavioral state and
cognitive processes (16). In previous work (8,
19), we characterized the ongoing activity measured optically,
showing that it is strongly correlated with the local EEG and is
composed of highly structured, ever-changing patterns of coherent
activity. Taken together, these findings indicate that old notions of
what is "noise" in brain activity may have to be revised. Because
the ongoing activity is often very large, we would expect it to play a
major role in cortical function. It may provide the neuronal substrate
for the dependence of sensory information processing on context and on
behavioral and conscious states. Indeed, the ongoing activity also
affects the behavior of the awake macaque monkey: The reaction time in
an arm-reaching paradigm could be predicted from the ongoing activity
preceding the arm movement (20).
REFERENCES AND NOTES
-
P. H. Schiller,
B. L. Finlay,
S. F. Volman,
Brain Res.
105,
347
(1976)
[Medline];
P. Heggelund and
K. Albus,
Exp. Brain Res.
32,
197
(1978)
[Medline];
R. P. Scobey and
A. J. Gabor,
ibid.
77,
398
(1989)
[Medline].
-
R. Vogels,
W. Spileers,
G. A. Orban,
Exp. Brain Res.
77,
432
(1989)
[Medline];
R. J. Snowden,
S. Treue,
R. A. Andersen,
ibid.
88,
389
(1992)
[Medline];
W. R. Softky and
C. Koch,
J. Neurosci.
13,
334
(1993)
[Medline].
-
W. A. Rosenblith, Ed., Processing Neuroelectric
Data (MIT Press, Cambridge, MA, 1959);
G. L. Gerstein,
Science
131,
1811
(1960)
.
-
E. R. John,
Science
177,
850
(1972)
[Medline];
G. L. Shaw,
E. Harth,
A. B. Scheibel,
Exp. Neurol.
77,
324
(1982)
[Medline];
K. H. Britten,
M. N. Shadlen,
W. T. Newsome,
W. A. Movshon,
J. Neurosci.
12,
4745
(1992)
[Medline].
-
Z. F. Mainen and
T. J. Sejnowski,
Science
268,
1503
(1995)
[Medline].
E. Hartveit and
P. Heggelund,
J. Neurophysiol.
72,
1278
(1994)
[Medline];
F. Mechler,
R. Shapley,
M. J. Hawken,
Soc. Neurosci. Abstr.
21,
22
(1995); G. R. Holt, W. R. Softky, C. Koch, R. J. Douglas, ibid., p. 22;
W. S. Geisler
and
D. G. Albrecht,
Vision Res.
35,
2723
(1995)
;
H. E. Wheat,
A. W. Goodwin,
A. S. Browning,
J. Neurosci.
15,
5582
(1995)
[Medline];
M. N. Shadlen,
K. H. Britten,
W. T. Newsome,
J. A. Movshon,
ibid.
16,
1486
(1996)
[Medline].
-
M. N. Shadlen and
W. T. Newsome,
Curr. Opin. Neurobiol.
4,
569
(1994)
[Medline];
D. Ferster and
N. Spruston,
Science
270,
756
(1995)
[Medline].
-
A. Arieli,
D. Shoham,
R. Hildesheim,
A. Grinvald,
J. Neurophysiol.
73,
2072
(1995)
[Medline].
-
Preliminary results were presented in abstract form [
A. Arieli,
A. Sterkin,
A. Grinvald,
A. Aertsen,
Soc. Neurosci. Abstr.
21,
772
(1995)].
-
Surgery was performed under aseptic conditions and deep
anesthesia. All procedures were carried out in accordance with the
National Institutes of Health and Weizmann Institute regulations for
animal care.
-
A. Grinvald,
A. Manker,
M. Segal,
J. Physiol.
333,
269
(1982);
A. Grinvald,
L. Anglister,
J. A. Freeman,
R. Hildesheim,
A. Manker,
Nature
308,
848
(1984)
;
H.
S. Orbach,
L. B. Cohen,
A. Grinvald,
J. Neurosci.
5,
1886
(1985)
[Medline];
A. Grinvald,
R. D. Frostig,
E. Lieke,
R. Hildesheim,
Physiol. Rev.
68,
1285
(1988)
[Medline].
-
A. Grinvald,
E. E. Lieke,
R. D. Frostig,
R. Hildesheim,
J. Neurosci.
14,
2545
(1994)
[Medline].
-
Components not related to the neuronal activity were removed
from the optical signals. Those components originate from changes in
light absorption by hemoglobin with every heartbeat or from movement of
the cortical tissue as a result of heart pulsation and respiration.
Using the fact that the heartbeat artifact is synchronized with the
electrocardiogram (ECG), we eliminated the artifact by subtracting the
ECG-triggered average optical signal from the raw data at each
heartbeat (8, 14). This "cleaning" procedure was
recently improved by analogous elimination of the respiratory wave. The
two series of images that were thus removed from the data are referred
to as the artifact. Before application of this cleaning procedure, the
average correlation coefficient between the optical images and the
artifact was 0.8; after this procedure, the correlation dropped to 0.02 (A. Sterkin et al., in preparation).
-
G. Gratton and
P. M. Corballis,
Psychophysiology
32,
292
(1995)
[Medline].
-
R. Cooper,
A. L. Winter,
H. J. Crow,
W. G. Walter,
Electroencephalogr. Clin. Neurophysiol.
18,
217
(1965)
;
R. Elul,
Int. Rev. Neurobiol.
15,
227
(1972)
;
O. D. Creutzfeldt and J. Houchin, in Electrical Activity from the
Neuron to the EEG and EMG, vol. 2C of Handbook of
Electroencephalography and Clinical Neurophysiology, O. D. Creutzfeldt, Ed. (Elsevier, Amsterdam, 1974), pp. 5-54.
-
A. S. Gevins and
R. E. Schaffer,
Crit. Rev. Bioeng.
1,
113
(1980)
; D. Regan, Human Brain
Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in
Science and Medicine (Elsevier, New York, 1989).
-
M. Abeles,
H. Bergman,
E. Margalit,
E. Vaadia,
J. Neurophysiol.
70,
1629
(1993)
[Medline];
W. Blair,
C. Koch,
W. T. Newsome,
K. H. Britten,
Soc. Neurosci. Abstr.
20,
1279
(1994).
-
Part of the linearity is presumably caused by the fact that
these measurements reflect synaptic population activity. Therefore,
they are subject to a linearizing effect similar to that reported
previously for evoked potentials [
H. Spekreijse and
L. H. van der
Tweel,
Nature
205,
913
(1965)
]. For single-neuron
activity we would expect a more prominent nonlinear behavior (for
example, related to the firing threshold). The observed reduction in
correlation between ongoing activity and single-neuron firing rate
(Fig. 2D) is consistent with this.
-
A. Arieli, in Information Processing in the
Cortex: Experiments and Theory, A. Aertsen and V. Braitenberg,
Eds. (Springer-Verlag, Berlin, 1992), pp. 123-138.
-
___ et al., Soc. Neurosci. Abstr.,
in press.
- We thank
E. Ahissar, Y. Fregnac, R. Malach, D. Sagi,
W. von Seelen, M. Segal, D. Shoham, I. Steinberg,
S. Ullman, and E. Vaadia
for their constructive comments. Supported in part by grants from the
Wolfson Foundation; the Israel Science Foundation, administered by the
Israel Academy of Sciences and Humanities; the Minerva Foundation,
Munich, Germany; and from the Human Frontier Science
Program.
19 March 1996; accepted 16 July
1996
This article has been cited by other articles:
- Linkenkaer-Hansen, K., Nikulin, V. V., Palva, S., Ilmoniemi, R. J., Palva, J. M.
(2004). Prestimulus Oscillations Enhance Psychophysical Performance in Humans. J. Neurosci.
24: 10186-10190
[Abstract]
[Full Text]
- Sachdev, R. N. S., Ebner, F. F., Wilson, C. J.
(2004). Effect of Subthreshold Up and Down States on the Whisker-Evoked Response in Somatosensory Cortex. J. Neurophysiol.
92: 3511-3521
[Abstract]
[Full Text]
- Paninski, L., Shoham, S., Fellows, M. R., Hatsopoulos, N. G., Donoghue, J. P.
(2004). Superlinear Population Encoding of Dynamic Hand Trajectory in Primary Motor Cortex. J. Neurosci.
24: 8551-8561
[Abstract]
[Full Text]
- Deweese, M. R., Zador, A. M.
(2004). Shared and Private Variability in the Auditory Cortex. J. Neurophysiol.
92: 1840-1855
[Abstract]
[Full Text]
- Goldberg,
J. A., Rokni, U., Boraud, T., Vaadia, E., Bergman, H. (2004). Spike
Synchronization in the Cortex-Basal Ganglia Networks of Parkinsonian
Primates Reflects Global Dynamics of the Local Field Potentials. J. Neurosci.
24: 6003-6010
[Abstract]
[Full Text]
- Beer, R. D.
(2003). The Dynamics of Active Categorical Perception in an Evolved Model Agent. Adaptive Behavior
11: 209-243
[Abstract]
- Vanhatalo, S., Palva, J. M., Holmes, M. D., Miller, J. W., Voipio, J., Kaila, K.
(2004). Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc. Natl. Acad. Sci. U. S. A.
101: 5053-5057
[Abstract]
[Full Text]
- Kuhn, A., Aertsen, A., Rotter, S.
(2004). Neuronal Integration of Synaptic Input in the Fluctuation-Driven Regime. J. Neurosci.
24: 2345-2356
[Abstract]
[Full Text]
- Masuda, N., Aihara, K.
(2004). Self-Organizing Dual Coding Based on Spike-Time-Dependent Plasticity. Neural Comput
16: 627-663
[Abstract]
[Full Text]
- Petersen, C. C. H., Hahn, T. T. G., Mehta, M., Grinvald, A., Sakmann, B.
(2003). Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. U. S. A.
100: 13638-13643
[Abstract]
[Full Text]
- Kruglikov, S. Y., Schiff, S. J.
(2003). Interplay of Electroencephalogram Phase and Auditory-Evoked Neural Activity. J. Neurosci.
23: 10122-10127
[Abstract]
[Full Text]
- Murphy, B. K., Miller, K. D.
(2003). Multiplicative Gain Changes Are Induced by Excitation or Inhibition Alone. J. Neurosci.
23: 10040-10051
[Abstract]
[Full Text]
- Laufs,
H., Krakow, K., Sterzer, P., Eger, E., Beyerle, A., Salek-Haddadi, A.,
Kleinschmidt, A. (2003). Electroencephalographic signatures of
attentional and cognitive default modes in spontaneous brain activity
fluctuations at rest. Proc. Natl. Acad. Sci. U. S. A.
100: 11053-11058
[Abstract]
[Full Text]
- Ariav,
G., Polsky, A., Schiller, J. (2003). Submillisecond Precision of the
Input-Output Transformation Function Mediated by Fast Sodium Dendritic
Spikes in Basal Dendrites of CA1 Pyramidal Neurons. J. Neurosci.
23: 7750-7758
[Abstract]
[Full Text]
- Masuda, N., Aihara, K.
(2003). Ergodicity of Spike Trains: When Does Trial Averaging Make Sense?. Neural Comput
15: 1341-1372
[Abstract]
[Full Text]
- Derdikman, D., Hildesheim, R., Ahissar, E., Arieli, A., Grinvald, A.
(2003). Imaging Spatiotemporal Dynamics of Surround Inhibition in the Barrels Somatosensory Cortex. J. Neurosci.
23: 3100-3105
[Abstract]
[Full Text]
- Leopold, D. A., Murayama, Y., Logothetis, N. K.
(2003). Very Slow Activity Fluctuations in Monkey Visual Cortex: Implications for Functional Brain Imaging. Cereb Cortex
13: 422-433
[Abstract]
[Full Text]
- Massimini,
M., Rosanova, M., Mariotti, M. (2003). EEG Slow (~1 Hz) Waves Are
Associated With Nonstationarity of Thalamo-Cortical Sensory Processing
in the Sleeping Human. J. Neurophysiol.
89: 1205-1213
[Abstract]
[Full Text]
- Masuda, N., Aihara, K.
(2003). Duality of Rate Coding and Temporal Coding in Multilayered Feedforward Networks. Neural Comput
15: 103-125
[Abstract]
[Full Text]
- Maass, W., Natschlager, T., Markram, H.
(2002). Real-Time Computing Without Stable States: A New Framework for Neural Computation Based on Perturbations. Neural Comput
14: 2531-2560
[Abstract]
[Full Text]
- Hansel, D., van Vreeswijk, C.
(2002). How Noise Contributes to Contrast Invariance of Orientation Tuning in Cat Visual Cortex. J. Neurosci.
22: 5118-5128
[Abstract]
[Full Text]
- Lutz,
A., Lachaux, J.-P., Martinerie, J., Varela, F. J. (2002). Guiding the
study of brain dynamics by using first-person data: Synchrony patterns
correlate with ongoing conscious states during a simple visual task. Proc. Natl. Acad. Sci. U. S. A.
99: 1586-1591
[Abstract]
[Full Text]
- Miller, K. D., Troyer, T. W.
(2002). Neural Noise Can Explain Expansive, Power-Law Nonlinearities in Neural Response Functions. J. Neurophysiol.
87: 653-659
[Abstract]
[Full Text]
- HAMEROFF, S.
(2001). Consciousness, the Brain, and Spacetime Geometry. Annals NYAS Online
929: 74-104
[Abstract]
[Full Text]
- Fuhrmann, G., Segev, I., Markram, H., Tsodyks, M.
(2002). Coding of Temporal Information by Activity-Dependent Synapses. J. Neurophysiol.
87: 140-148
[Abstract]
[Full Text]
- Grun, S., Diesmann, M., Aertsen, A.
(2002). Unitary Events in Multiple Single-Neuron Spiking Activity: II. Nonstationary Data. Neural Comput
14: 81-119
[Abstract]
[Full Text]
- Araki,
O., Aihara, K. (2001). Dual Information Representation with Stable
Firing Rates and Chaotic Spatiotemporal Spike Patterns in a Neural
Network Model. Neural Comput
13: 2799-2822
[Abstract]
[Full Text]
- Amemori, K.-i., Ishii, S.
(2001). Gaussian Process Approach to Spiking Neurons for Inhomogeneous Poisson Inputs. Neural Comput
13: 2763-2797
[Abstract]
[Full Text]
- Migliore, M., Messineo, L., Cardaci, M., Ayala, G. F.
(2001). Quantitative Modeling of Perception and Production of Time Intervals. J. Neurophysiol.
86: 2754-2760
[Abstract]
[Full Text]
- Hasty, J., Collins, J. J., Wiesenfeld, K., Grigg, P.
(2001). Wavelets of Excitability in Sensory Neurons. J. Neurophysiol.
86: 2097-2101
[Abstract]
[Full Text]
- Beierholm, U., Nielsen, C. D., Ryge, J., Alstrom, P., Kiehn, O.
(2001). Characterization of Reliability of Spike Timing in Spinal Interneurons During Oscillating Inputs. J. Neurophysiol.
86: 1858-1868
[Abstract]
[Full Text]
- Lauritzen, T. Z., Krukowski, A. E., Miller, K. D.
(2001). Local Correlation-Based Circuitry Can Account for Responses to Multi-Grating Stimuli in a Model of Cat V1. J. Neurophysiol.
86: 1803-1815
[Abstract]
[Full Text]
- Steriade, M.
(2001). Impact of Network Activities on Neuronal Properties in Corticothalamic Systems. J. Neurophysiol.
86: 1-39
[Abstract]
[Full Text]
- Bair, W., Zohary, E., Newsome, W. T.
(2001). Correlated Firing in Macaque Visual Area MT: Time Scales and Relationship to Behavior. J. Neurosci.
21: 1676-1697
[Abstract]
[Full Text]
- Linkenkaer-Hansen, K., Nikouline, V. V., Palva, J. M., Ilmoniemi, R. J.
(2001). Long-Range Temporal Correlations and Scaling Behavior in Human Brain Oscillations. J. Neurosci.
21: 1370-1377
[Abstract]
[Full Text]
- Gluckman, B. J., Nguyen, H., Weinstein, S. L., Schiff, S. J.
(2001). Adaptive Electric Field Control of Epileptic Seizures. J. Neurosci.
21: 590-600
[Abstract]
[Full Text]
- von Stein, A., Chiang, C., Konig, P.
(2000). Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. U. S. A.
97: 14748-14753
[Abstract]
[Full Text]
- Faure, P., Kaplan, D., Korn, H.
(2000). Synaptic Efficacy and the Transmission of Complex Firing Patterns Between Neurons. J. Neurophysiol.
84: 3010-3025
[Abstract]
[Full Text]
- Brody, C. D.
(1999). Correlations Without Synchrony. Neural Comput
11: 1537-1551
[Abstract]
[Full Text]
- Shtoyerman,
E., Arieli, A., Slovin, H., Vanzetta, I., Grinvald, A. (2000).
Long-Term Optical Imaging and Spectroscopy Reveal Mechanisms Underlying
the Intrinsic Signal and Stability of Cortical Maps in V1 of Behaving
Monkeys. J. Neurosci.
20: 8111-8121
[Abstract]
[Full Text]
- Kimura,
A., Pavlides, C. (2000). Long-Term Potentiation/Depotentiation Are
Accompanied by Complex Changes in Spontaneous Unit Activity in the
Hippocampus. J. Neurophysiol.
84: 1894-1906
[Abstract]
[Full Text]
- Hô, N., Destexhe, A.
(2000). Synaptic Background Activity Enhances the Responsiveness of Neocortical Pyramidal Neurons. J. Neurophysiol.
84: 1488-1496
[Abstract]
[Full Text]
- Harsch,
A., Robinson, H. P. C. (2000). Postsynaptic Variability of Firing in
Rat Cortical Neurons: The Roles of Input Synchronization and Synaptic
NMDA Receptor Conductance. J. Neurosci.
20: 6181-6192
[Abstract]
[Full Text]
- Jancke, D.
(2000). Orientation Formed by a Spot's Trajectory: A Two-Dimensional Population Approach in Primary Visual Cortex. J. Neurosci.
20: 86R-86
[Abstract]
[Full Text]
- Furukawa, S., Xu, L., Middlebrooks, J. C.
(2000). Coding of Sound-Source Location by Ensembles of Cortical Neurons. J. Neurosci.
20: 1216-1228
[Abstract]
[Full Text]
- Tsodyks, M., Kenet, T., Grinvald, A., Arieli, A.
(1999). Linking Spontaneous Activity of Single Cortical Neurons and the Underlying Functional Architecture. Science
286: 1943-1946
[Abstract]
[Full Text]
- Kisley, M. A., Gerstein, G. L.
(1999). Trial-to-Trial Variability and State-Dependent Modulation of Auditory-Evoked Responses in Cortex. J. Neurosci.
19: 10451-10460
[Abstract]
[Full Text]
- Schiff, N. D., Purpura, K. P., Victor, J. D.
(1999). Gating of Local Network Signals Appears as Stimulus-Dependent Activity Envelopes in Striate Cortex. J. Neurophysiol.
82: 2182-2196
[Abstract]
[Full Text]
- Jancke,
D., Erlhagen, W., Dinse, H. R., Akhavan, A. C., Giese, M., Steinhage,
A., Schöner, G. (1999). Parametric Population Representation of Retinal
Location: Neuronal Interaction Dynamics in Cat Primary Visual Cortex. J. Neurosci.
19: 9016-9028
[Abstract]
[Full Text]
- Whitsel, B. L., Favorov, O., Delemos, K. A., Lee, C.-J., Tommerdahl, M., Essick, G. K., Nakhle, B.
(1999). SI Neuron Response Variability Is Stimulus Tuned and NMDA Receptor Dependent. J. Neurophysiol.
81: 2988-3006
[Abstract]
[Full Text]
- Wu, J.-y., Guan, L., Tsau, Y.
(1999). Propagating Activation during Oscillations and Evoked Responses in Neocortical Slices. J. Neurosci.
19: 5005-5015
[Abstract]
[Full Text]
- Brecht, M., Singer, W., Engel, A. K.
(1999). Patterns of Synchronization in the Superior Colliculus of Anesthetized Cats. J. Neurosci.
19: 3567-3579
[Abstract]
[Full Text]
- Azouz, R., Gray, C. M.
(1999). Cellular Mechanisms Contributing to Response Variability of Cortical Neurons In Vivo. J. Neurosci.
19: 2209-2223
[Abstract]
[Full Text]
- deCharms, R. C.
(1998). Information coding in the cortex by independent or coordinated populations. Proc. Natl. Acad. Sci. U. S. A.
95: 15166-15168
[Full Text]
- Nakagawa, H., Matsumoto, N.
(1998). ON and OFF Channels of the Frog Optic Tectum Revealed by Current Source Density Analysis. J. Neurophysiol.
80: 1886-1899
[Abstract]
[Full Text]
- Hunter, J. D., Milton, J. G., Thomas, P. J., Cowan, J. D.
(1998). Resonance Effect for Neural Spike Time Reliability. J. Neurophysiol.
80: 1427-1438
[Abstract]
[Full Text]
- Shadlen, M. N., Newsome, W. T.
(1998). The Variable Discharge of Cortical Neurons: Implications for Connectivity, Computation, and Information Coding. J. Neurosci.
18: 3870-3896
[Abstract]
[Full Text]
- Berry, M. J., Warland, D. K., Meister, M.
(1997). The structure and precision of retinal spike trains. Proc. Natl. Acad. Sci. U. S. A.
94: 5411-5416
[Abstract]
[Full Text]
- Gur, M., Beylin, A., Snodderly, D. M.
(1997). Response Variability of Neurons in Primary Visual Cortex (V1) of Alert Monkeys. J. Neurosci.
17: 2914-2920
[Abstract]
[Full Text]
- Lee, D., Port, N. L., Kruse, W., Georgopoulos, A. P.
(1998). Variability and Correlated Noise in the Discharge of Neurons in Motor and Parietal Areas of the Primate Cortex. J. Neurosci.
18: 1161-1170
[Abstract]
[Full Text]
- Roerig, B., Katz, L. C.
(1997). Modulation of Intrinsic Circuits by Serotonin 5-HT3 Receptors in Developing Ferret Visual Cortex. J. Neurosci.
17: 8324-8338
[Abstract]
[Full Text]
Volume 273,
Number 5283,
Issue of 27 Sep 1996,
pp. 1868-1871.
Copyright © 1996 by The American Association for the Advancement of Science. All rights reserved.
|


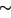 5 × 10
5 × 10 5). The small square in the first image
marks the site, above the microelectrode, from which the optical traces
(2a,b) were taken. Note the large variability in the evoked response,
also reflected in the LFP (3a,b) and single-neuron spike trains (4a,b),
both recorded simultaneously with the optical signals. The absence of
slow components in the LFP is due to high-pass filtering above 3 Hz.
(B) Average evoked response: The optical images and
signals, LFP, and single-unit activity were averaged, triggered on the
onset of 34 visual stimuli (drifting full-field grating) in the
preferred orientation of the recorded unit.
5). The small square in the first image
marks the site, above the microelectrode, from which the optical traces
(2a,b) were taken. Note the large variability in the evoked response,
also reflected in the LFP (3a,b) and single-neuron spike trains (4a,b),
both recorded simultaneously with the optical signals. The absence of
slow components in the LFP is due to high-pass filtering above 3 Hz.
(B) Average evoked response: The optical images and
signals, LFP, and single-unit activity were averaged, triggered on the
onset of 34 visual stimuli (drifting full-field grating) in the
preferred orientation of the recorded unit.



 I], last column): a single-trial estimate
of the reproducible response to this particular stimulus. These net
patterns are very similar, whereas the measured patterns (second
column) are variable, suggesting that "removal" of the ongoing
activity from the measured response does markedly reduce the response
variability. We do not know if the lack of a perfect match among the
net patterns should be attributed solely to the change of ongoing
activity from the initial state or whether, in addition, it reflects
deviations from the simplified, linear model.
I], last column): a single-trial estimate
of the reproducible response to this particular stimulus. These net
patterns are very similar, whereas the measured patterns (second
column) are variable, suggesting that "removal" of the ongoing
activity from the measured response does markedly reduce the response
variability. We do not know if the lack of a perfect match among the
net patterns should be attributed solely to the change of ongoing
activity from the initial state or whether, in addition, it reflects
deviations from the simplified, linear model.
